Chiral separation of amines with N-benzoxycarbonylglycyl-L-proline as selector in non-aqueous capillary electrophoresis using methanol and 1,2-dichloroethane in the background electrolyte.
Ylva Hedeland, Mikael Hedeland, Ulf Bondesson, Curt Pettersson
文献索引:J. Chromatogr. A. 984(2) , 261-71, (2003)
全文:HTML全文
摘要
N-Benzoxycarbonylglycyl-L-proline (L-ZGP) has been introduced as a chiral selector for enantioseparation of amines in non-aqueous capillary electrophoresis. Methanol mixed with different proportions of dichloromethane, 1,2-dichloroethane or 2-propanol containing L-ZGP and ammonium acetate was used as the background electrolyte. Enantioseparation of different types of pharmacologically active amines was performed, e.g. the local anaesthetic bupivacaine and the beta-adrenoceptor blocking agent pindolol. Addition of the solvents (dichloromethane, 1,2-dichloroethane or 2-propanol) gave an improved chiral separation partly due to a distinct decrease in the electroosmotic flow. The use of 1,2-dichloroethane in the background electrolyte gave higher precision in migration time (RSD 2.2%) compared to the systems containing dichloromethane. An enantiomeric separation of mepivacaine was performed within 72 s by use of short-end injection with an effective capillary length of 8.5 cm.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
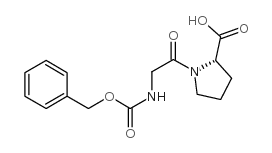 |
N-苄氧羰基甘氨酰-L-脯氨酸
CAS:1160-54-9 |
C15H18N2O5 |
|
Determination of (R)- and (S)-propranolol in plasma by high-...
[J. Chromatogr. A. 494 , 157-71, (1989)] |
|
Enantioselective high-performance liquid chromatographic det...
[J. Chromatogr. A. 620(2) , 217-24, (1993)] |
|
Enantiomeric separation of basic drugs using N-benzyloxycarb...
1995-06-30 [J. Chromatogr. A. 705(2) , 275-87, (1995)] |
|
Possible involvement of aminopeptidase, an ecto-enzyme, in t...
1985-10-30 [Biochim. Biophys. Acta 847(1) , 67-76, (1985)] |
|
Structures of prolyl oligopeptidase substrate/inhibitor comp...
2001-01-12 [J. Biol. Chem. 276(2) , 1262-6, (2001)] |