Synthesis and evaluation of antitumor activity of novel 2-[Nmethyl-N-(4-methyl-1,3-benzothiazol-2-yl)aminomethyl]-5,8-diacyloxy-1,4-naphthoquinones.
Jikang Yoo, Hyun-Suk Choi, Cheol-Hee Choi, Yongseog Chung, Bok Hee Kim, Hoon Cho
文献索引:Arch. Pharm. Res. 31(2) , 142-7, (2008)
全文:HTML全文
摘要
A series of nine new compounds bridged by acyl groups at the 5,8-dihydroxyl group of DHNQ were synthesized and their cytotoxic activity against L1210 and P388 cancer cells was examined. Their antitumor action in mice bearing S-180 cells in the peritoneal cavity was also assessed. Increasing the size of the acyl group (compounds 7-9) up to propyl increased the antitumor activity (T/C value), whereas the cytotoxicity of these compounds was comparable against L1210 (lymphocytic leukemia) and P388 (lymphoid neoplasm) cancer cells. Further increasing in the chain length (compounds 11-15) decreased the potency. Thus, acyl group chains of three carbon atoms is optimal for antitumor activity. The most potent compound of this series was 2-[N-methyl-N-(4-methyl-1,3-benzothiazol-2-yl)aminomethyl]-5,8-dipropylcarbonyloxy-1,4-naphthoquinone (compound 9) with a T/C (%) value of 354.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
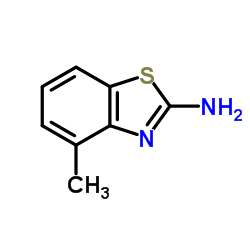 |
2-氨基-4-甲基苯并噻唑
CAS:1477-42-5 |
C8H8N2S |
|
Ultra-high performance liquid chromatography tandem high-res...
2015-06-01 [Environ. Sci. Pollut. Res. Int. 22 , 8288-95, (2015)] |
|
SYNTHESIS, STRUCTURAL CHARACTERIZATION AND ANTIMICROBIAL ACT...
[Int. J. Pharm. Biol. Sci. 2(3) , 430-39, (2011)] |
|
Experimental and theoretical surface enhanced Raman scatteri...
2005-12-01 [J. Phys. Chem. B 109(47) , 22536-44, (2005)] |
|
Biotic and abiotic degradation of pesticide Dufulin in soils...
2014-03-01 [Environ. Sci. Pollut. Res. Int. 21(6) , 4331-42, (2014)] |