Rat liver S-adenosylhomocysteinase. Spectrophotometric study of coenzyme binding.
T Gomi, Y Takata, M Fujioka
文献索引:Biochim. Biophys. Acta 994 , 172-179, (1989)
全文:HTML全文
摘要
Rat liver S-adenosylhomocysteinase, a homotetramer, was resolved by treatment with acid ammonium sulfate into apoenzyme and NAD. The apoenzyme thus prepared retained a tetrameric structure but differed in the mobility on nondenaturing polyacrylamide gel electrophoresis. The inactive apoenzyme was reactivated upon incubation with NAD. The restoration of activity paralleled with the tight binding of NAD to apoenzyme, and full activity was obtained when 4 mol of NAD were bound per mol of apoenzyme. The kinetics of reconstitution were apparently biphasic and suggest the existence of two conformers in a slow equilibrium, one of which binds the coenzyme rapidly while the other does so very slowly, if at all. In addition to NAD, apoadenosylhomocysteinase tightly bound nicotinamide hypoxanthine dinucleotide, 3-acetylpyridine adenine dinucleotide and nicotinic acid-adenine dinucleotide. NADP was not bound. Catalytic activity was found only with the enzyme reconstituted with NAD or nicotinamide hypoxanthine dinucleotide. The spectral change observed on interaction of apoadenosylhomocysteinase with NAD was similar to those seen with adenine nucleotides, and was largely approximated by the addition of dioxane to aqueous solutions of adenine nucleotides. By comparison of the difference spectra, it is suggested that the adenine portion of the coenzyme is bound in the hydrophobic pocket of the protein, and that the binding is accompanied by perturbation of tryptophan residue of the protein.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
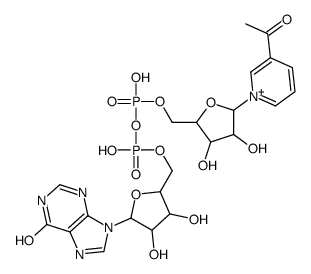 |
3-乙酰吡啶次黄嘌呤二核苷酸
CAS:4002-09-9 |
C22H28N5O15P2+ |
|
NAD+ analogs substituted in the purine base as substrates fo...
1996-11-11 [FEBS Lett. 397 , 17-21, (1996)] |
|
Interactions of nicotinamide-adenine dinucleotide phosphate ...
1987-07-15 [Biochem. J. 245 , 407, (1987)] |
|
Kinetic mechanism of the endogenous lactate dehydrogenase ac...
1991-02-01 [Arch. Biochem. Biophys. 284 , 285-291, (1991)] |