Co-oxidation of 2-bromohydroquinone by renal prostaglandin synthase. Modulation of prostaglandin synthesis by 2-bromohydroquinone and glutathione.
S S Lau, T J Monks
文献索引:Drug Metab. Dispos. 15(6) , 801-7, (1987)
全文:HTML全文
摘要
Homogenates from rat renal papillae, a rich source of the prostaglandin (PG) H synthase system (PHS), metabolized [14C]2-bromohydroquinone, in the presence of arachidonic acid, to products which are covalently bound to protein. The co-oxidation of 2-bromohydroquinone caused a concentration-dependent stimulation in 6-keto-PGF1 alpha, thromboxane B2, PGF2 alpha, PGE2, and PGD2 formation. Glutathione (1 mM) caused a decrease in prostaglandin formation and inhibited the arachidonic acid-supported covalent binding of [14C]2-bromohydroquinone with the concomitant formation of [14C]2-bromohydroquinone-glutathione conjugates, oxidized glutathione, and an increase in the recovery of [14C]2-bromohydroquinone. NADPH also inhibited [14C]2-bromohydroquinone covalent binding, probably by reduction of the semiquinone radical back to the hydroquinone. Indomethacin and aspirin, inhibitors of the cyclooxygenase component of PHS, and propylthiouracil and methimazole, inhibitors of the hydroperoxidase component of PHS, inhibited the arachidonic acid-supported covalent binding of [14C]2-bromohydroquinone by 94%, 52%, 78%, and 79% respectively. These data suggest that 1) renal PHS may play a role in activating the nephrotoxin, 2-bromohydroquinone, and that 2) xenobiotic metabolism and its subsequent effects on glutathione levels can modulate renal prostaglandin synthesis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
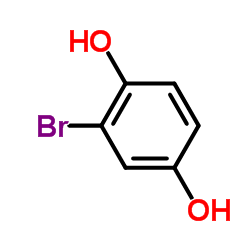 |
2-溴对苯二酚
CAS:583-69-7 |
C6H5BrO2 |
|
Dihydroxylated mercapturic acid metabolites of bromobenzene.
1992-01-01 [Chem. Res. Toxicol. 5(4) , 561-7, (1992)] |
|
Inhibition of respiration in rabbit proximal tubules by brom...
1986-01-01 [Adv. Exp. Med. Biol. 197 , 911-7, (1986)] |
|
Cellular toxicity of bromobenzene and bromobenzene metabolit...
1986-05-01 [J. Pharmacol. Exp. Ther. 237(2) , 456-61, (1986)] |
|
Synthesis and nephrotoxicity of 6-bromo-2,5-dihydroxy-thioph...
1988-07-01 [Mol. Pharmacol. 34(1) , 15-22, (1988)] |
|
2-Bromohydroquinone-induced toxicity to rabbit renal proxima...
1989-06-01 [Toxicol. Appl. Pharmacol. 99(1) , 11-8, (1989)] |