Chiral alcohol production by NADH-dependent phenylacetaldehyde reductase coupled with in situ regeneration of NADH.
Nobuya Itoh, Michiko Matsuda, Mariko Mabuchi, Tohru Dairi, Jincun Wang
文献索引:Eur. J. Biochem. 269(9) , 2394-402, (2002)
全文:HTML全文
摘要
Phenylacetaldehyde reductase (PAR) produced by styrene-assimilating Corynebacterium strain ST-10 was used to synthesize chiral alcohols. This enzyme with a broad substrate range reduced various prochiral aromatic ketones and beta-ketoesters to yield optically active secondary alcohols with an enantiomeric purity of more than 98% enantiomeric excess (e.e.). The Escherichia coli recombinant cells which expressed the par gene could efficiently produce important pharmaceutical intermediates; (R)-2-chloro-1-(3-chlorophenyl)ethanol (28 mg.mL-1) from m-chlorophenacyl chloride, ethyl (R)-4-chloro-3-hydroxy butanoate) (28 mg.mL-1) from ethyl 4-chloro-3-oxobutanoate and (S)-N-tert-butoxycarbonyl(Boc)-3-pyrrolidinol from N-Boc-3-pyrrolidinone (51 mg.mL-1), with more than 86% yields. The high yields were due to the fact that PAR could concomitantly reproduce NADH in the presence of 3-7% (v/v) 2-propanol in the reaction mixture. This biocatalytic process provided one of the best asymmetric reductions ever reported.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
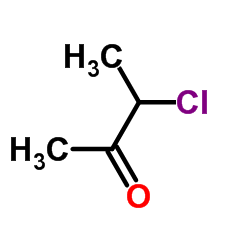 |
3-氯-2-丁酮
CAS:4091-39-8 |
C4H7ClO |
|
Substrate specificity and enantioselectivity of 4-hydroxyace...
2003-01-01 [Appl. Environ. Microbiol. 69(1) , 419-26, (2003)] |
|
Synthesis and activity of ruthenium olefin metathesis cataly...
2008-02-20 [J. Am. Chem. Soc. 130(7) , 2234-45, (2008)] |
|
Reaction of α-chloroketones with 1, 4-dianion of acetop...
[Tetrahedron Lett. 25(40) , 4529-32, (1984)] |
|
Reactions of 3-Thiocyano-2-butanone. I. The Preparation of 2...
[J. Am. Chem. Soc. 74(7) , 1719-1720, (1952)] |