GABA analogues derived from 4-aminocyclopent-1-enecarboxylic acid.
Katherine E S Locock, Graham A R Johnston, Robin D Allan
文献索引:Neurochem. Res. 34(10) , 1698-703, (2009)
全文:HTML全文
摘要
The incorporation of extra binding groups onto known ligands is a powerful tool for the development of more potent and selective agents at target sites such as the GABA receptors. In the present work we have attempted to build on the activity of the know potent GABA(A) agonist 4-ACP-3-CA and its cis and trans saturated analogues CACP and TACP. We have investigated reactions to add thiol substituents to the alpha,beta-unsaturated system of 4-ACP-3-CA. The reaction was successful with a limited number of thiols but gave products of mixed stereochemistry. The resultant thioether amino acids were screened for activity at human recombinant alpha(1)beta(2) gamma(2L) GABA(A) receptors. The most interesting derivative was the benzylthioether which acted as an antagonist with an IC(50) of 42 microM for the inhibition of a GABA EC(50) dose (50 microM). This study has shown that GABA analogues derived by thiol addition to 4-aminocyclopent-1-enecarboxylic acid display interesting antagonist activity at the alpha(1)beta(2)gamma(2L) GABA(A) receptor.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
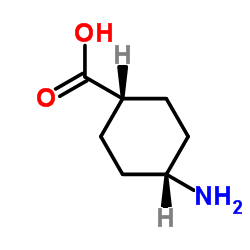 |
4-氨基环己烷-1-羧酸
CAS:3685-23-2 |
C7H13NO2 |
|
Opioid deltorphin C analogues containing cis- or trans-2- or...
1999-01-01 [Arzneimittelforschung 49(1) , 6-12, (1999)] |
|
cis-4-[[[(2-Chloroethyl)nitrosoamino]carbonyl]methylamino] c...
1984-01-01 [J. Med. Chem. 27(1) , 97-9, (1984)] |
|
Hydrogen-bonding patterns in cis-4-ammoniocyclohexanecarboxy...
2004-10-01 [Acta Crystallogr. C 60(Pt 10) , o759-61, (2004)] |
|
Cytotoxic T lymphocyte epitope analogues containing cis- or ...
2002-09-01 [Bioorg. Med. Chem. 10(9) , 3061-6, (2002)] |
|
Influence of conformationally constrained amino acids replac...
2007-01-01 [Protein Pept. Lett. 14(3) , 213-7, (2007)] |