Introduction of 3'-terminal nucleosides having a silyl-type linker into polymer supports without base protection.
Akihiro Ohkubo, Yasuhiro Noma, Katsufumi Aoki, Hirosuke Tsunoda, Kohji Seio, Mitsuo Sekine
文献索引:J. Org. Chem. 74(7) , 2817-23, (2009)
全文:HTML全文
摘要
New 3'-terminal deoxyribonucleoside-loading reagents having a silyl-type linker were developed. They were effectively introduced into polymer supports under the conditions of Huisgen [3 + 2] cycloaddition without base protection. Moreover, four unmodified DNA oligomers d[TACCTAAATCCAX] (X = T, A, C, and A) and a base-labile modified DNA 12mer d[A*C*T*C*C*GT*C*T*A*C*G] 16 (A* = 6-N-acetyl-8-aza-7-deaza-2'-deoxyadenosine, C* = 4-N-acetyl-2'-deoxyctydine, T* = 2-thio-T) were successfully synthesized by cleavage of the silyl-type linker using Bu(4)NF under neutral conditions in our N-unprotected phosphoramidite method. In this paper, we also report a new reaction of chlorination of cytosine base using 1,3-dichloro-5,5-dimethylhydantoin.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
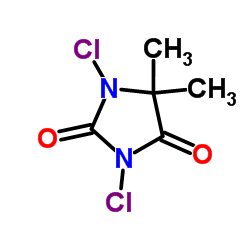 |
1,3-二氯-5,5-二甲基海因
CAS:118-52-5 |
C5H6Cl2N2O2 |
|
a-Chlorination of Acetophenones Using 1, 3-Dichloro-5, 5-Dim...
[Synth. Commun. 36(2) , 255-8, (2006)] |
|
Oxidation of urazoles with 1, 3-dihalo-5, 5-dimethylhydantoi...
[Synlett 5 , 761-4, (2005)] |
|
[Hygienic standard for dichlorantin and its transformation p...
1982-06-01 [Gig. Sanit. (6) , 76-8, (1982)] |
|
[Hygienic evaluation of the possible use of 1,3-dichloro-5,5...
1982-03-01 [Gig. Sanit. (3) , 11-3, (1982)] |
|
Evaluation of neutralized chemical agent identification sets...
1998-01-01 [J. Appl. Toxicol. 18(6) , 409-20, (1998)] |