Sequence selective formation of 1,N(6)-ethenoadenine in DNA by furan-conjugated probe.
Akio Kobori, Jumpei Morita, Masato Ikeda, Asako Yamayoshi, Akira Murakami
文献索引:Bioorg. Med. Chem. Lett. 19(13) , 3657-60, (2009)
全文:HTML全文
摘要
1,N(6)-Ethenoadenosine derivatives have been applied as fluorescence probes in various fields of biochemistry and molecular biology. We developed a 1,N(6)-ethenoadenosine-forming reaction at a target adenine in DNA duplex and applied it to a mutation diagnosis. Furan-derivatized oligodeoxyribonucleotides were synthesized and fluorescence properties were studied in the presence of complementary strand under oxidative conditions. Strong emissions at 430nm were observed in the presence of the complementary strand with an adenine in front of furan moiety.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
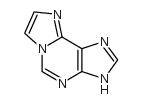 |
1,N⁶-乙基腺嘌呤
CAS:13875-63-3 |
C7H5N5 |
|
Measurement of urinary excretion of 5-hydroxymethyluracil in...
2005-03-15 [Toxicol. Lett. 155(3) , 403-10, (2005)] |
|
Highly mutagenic exocyclic DNA adducts are substrates for th...
2012-01-01 [PLoS ONE 7(12) , e51776, (2012)] |
|
Magnesium, essential for base excision repair enzymes, inhib...
2006-10-06 [J. Biol. Chem. 281(40) , 29525-32, (2006)] |
|
Modeling the chemical step utilized by human alkyladenine DN...
2011-10-12 [J. Am. Chem. Soc. 133(40) , 16258-69, (2011)] |
|
Structural insights by molecular dynamics simulations into d...
2002-09-01 [Nucleic Acids Res. 30(17) , 3778-87, (2002)] |