Base pairing and miscoding properties of 1,N(6)-ethenoadenine- and 3,N(4)-ethenocytosine-containing RNA oligonucleotides.
Alessandro Calabretta, Christian J Leumann
文献索引:Biochemistry 52(11) , 1990-7, (2013)
全文:HTML全文
摘要
Two RNA phosphoramidites containing the bases 1,N(6)-ethenoadenine (εA) and 3,N(4)-ethenocytosine (εC) were synthesized. These building blocks were incorporated into two 12-mer oligoribonucleotides for evaluation of the base pairing properties of these base lesions by UV melting curve (Tm) and circular dichroism measurements. The Tm data of the resulting duplexes with the etheno modifications opposing all natural bases showed a substantial destabilization compared to the corresponding natural duplexes, confirming their inability to form base pairs. The coding properties of these lesions were further investigated by introducing them into 31-mer oligonucleotides and assessing their ability to serve as templates in primer extension reactions with HIV, AMV, and MMLV reverse transcriptases (RT). Primer extension reactions showed complete arrest of the incorporation process using MMLV RT and AMV RT, while HIV RT preferentially incorporates dAMP opposite εA and dAMP as well as dTMP opposite εC. The properties of these RNA lesions are discussed in the context of its putative biological role.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
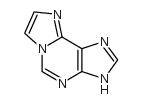 |
1,N⁶-乙基腺嘌呤
CAS:13875-63-3 |
C7H5N5 |
|
Measurement of urinary excretion of 5-hydroxymethyluracil in...
2005-03-15 [Toxicol. Lett. 155(3) , 403-10, (2005)] |
|
Highly mutagenic exocyclic DNA adducts are substrates for th...
2012-01-01 [PLoS ONE 7(12) , e51776, (2012)] |
|
Magnesium, essential for base excision repair enzymes, inhib...
2006-10-06 [J. Biol. Chem. 281(40) , 29525-32, (2006)] |
|
Modeling the chemical step utilized by human alkyladenine DN...
2011-10-12 [J. Am. Chem. Soc. 133(40) , 16258-69, (2011)] |
|
Structural insights by molecular dynamics simulations into d...
2002-09-01 [Nucleic Acids Res. 30(17) , 3778-87, (2002)] |