First total synthesis of (-)-achilleol B: reassignment of its relative stereochemistry.
Jesús F Arteaga, Victoriano Domingo, José F Quílez del Moral, Alejandro F Barrero
文献索引:Org. Lett. 10(9) , 1723-6, (2008)
全文:HTML全文
摘要
The first total synthesis of (-)-achilleol B was achieved using a convergent approach with a longest linear sequence of 14 steps. Three key steps were employed, including an enantioselective Robinson annelation for the construction of the bicyclic moiety. The monocyclic synthon was prepared through a Ti(III)-mediated cylization of a chiral monoepoxide obtained via asymmetric dihydroxylation of geranylacetone. The asymmetric preparation of these subunits also permitted us to achieve the enantioselective synthesis of elegansidiol, achilleol A, and farnesiferol B.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
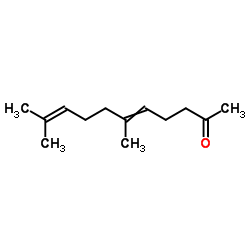 |
6,10-二甲基-5,9-十一双烯-2-酮
CAS:689-67-8 |
C13H22O |
|
An improved odor bait for monitoring populations of Aedes ae...
2015-01-01 [Parasit. Vectors 8 , 253, (2015)] |
|
Lycopene accumulation affects the biosynthesis of some carot...
2008-08-01 [J. Integr. Plant Biol. 50(8) , 991-6, (2008)] |
|
Ozone-initiated chemistry in an occupied simulated aircraft ...
2007-09-01 [Environ. Sci. Technol. 41(17) , 6177-84, (2007)] |
|
Identification of isoprenoid-type components in human expire...
1987-01-01 [Biomed. Chromatogr. 2(2) , 66-70, (1987)] |
|
Chemical composition and in vitro cytotoxic, genotoxic effec...
2012-05-01 [Bull. Environ. Contam. Toxicol. 88(5) , 666-71, (2012)] |