Identification of the catalytic residues of carboxylesterase from Arthrobacter globiformis by diisopropyl fluorophosphate-labeling and site-directed mutagenesis.
Masako Nishizawa, Yoshiyasu Yabusaki, Masaharu Kanaoka
文献索引:Biosci. Biotechnol. Biochem. 75(1) , 89-94, (2011)
全文:HTML全文
摘要
The role of amino acid residues in the enzymatic activity of carboxylesterase from Arthrobacter globiformis was analyzed by diisopropyl fluorophosphate (DFP) labeling and site-directed mutagenesis. The electrospray ionization mass spectrometric (ESI-MS) analysis of the esterase, covalently labeled by DFP, showed stoichiometric incorporation of the inhibitor into the enzyme. The further comparison of endopeptidase-digested fragments between native and DFP-labeled esterase by fast atom bombardment mass spectrometric (FAB-MS) analysis as well as site-directed mutagenesis indicated that Ser59 in the consensus sequence Ser-X-X-Lys, which is conserved exclusively in penicillin-binding proteins and some esterases, served as a catalytic nucleophile. In addition, the results obtained from analysis of the mutants at position 62 suggested the importance of the basic amino acid side chain at this position, and suggested the significance of this residue acting directly as a general base rather than its involvement in the maintenance of the optimum hydrogen-bonding network at the active site.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
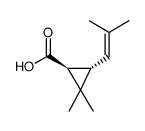 |
( )-反式-菊酸
CAS:4638-92-0 |
C10H16O2 |
|
[Determination of chrysanthemic acid and its ethyl ester in ...
1988-03-01 [Gig. Sanit. (3) , 42-3, (1988)] |
|
Characteristics and function of Alcaligenes sp. NBRC 14130 e...
2010-04-01 [J. Appl. Microbiol. 108(4) , 1263-70, (2010)] |
|
Gas chromatographic determination of racemic cis- and trans-...
[J. Chromatogr. A. 437(2) , 436-41, (1988)] |
|
Low pilot exposure to pyrethrin during ultra-low-volume (ULV...
2005-09-01 [J. Am. Mosq. Control Assoc. 21(3) , 291-5, (2005)] |
|
Bidirectional secretions from glandular trichomes of pyrethr...
2012-10-01 [Plant Cell 24(10) , 4252-65, (2012)] |