Studies on cardiac ingredients of plants. IX. Chemical transformation of proscillaridin by utilizing its 1,4-cycloadducts as key compounds and biological activities of their derivatives.
T Tanase, N Murakami, S Nagai, T Ueda, J Sakakibara, H Ando, Y Hotta, K Takeya
文献索引:Chem. Pharm. Bull. 40(2) , 327-32, (1992)
全文:HTML全文
摘要
Three aromatic compounds (2-4) possessing a carbomethoxyl group or a dimethoxyphthaloyl group, prepared by the Diels-Alder reaction of the cardiac glycoside, proscillaridin (1), with dimethyl acetylenedicarboxylate and methyl propiolate, were transformed into alcohols, carboxylic acids and amides. The biological activities of the resulting derivatives were evaluated by the use of Na+, K(+)-adenosine triphosphatase (Na+,K(+)-ATPase) from dog kidney and isolated guinea-pig papillary muscle. Although the biological activities of the resulting derivatives were less potent than that of 1, a para-substituted benzylalcohol (5), methylbenzamides (9a and 10a), and ethylbenzamides (9b and 10b) inhibited the activity of Na+,K(+)-ATPase almost as potently as naturally occurring cardiac glycosides such as digoxin and digitoxin.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
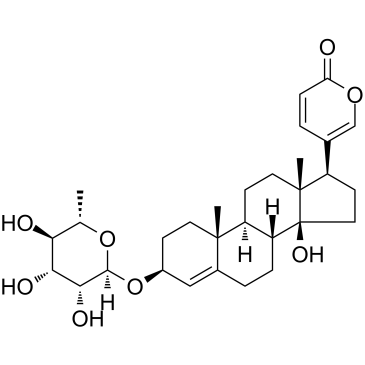 |
原海葱甙A
CAS:466-06-8 |
C30H42O8 |
|
Pulse pressure correlates in humans with a proscillaridin A ...
1996-05-01 [Hypertension 27(5) , 1073-8, (1996)] |
|
Cytotoxic effects of cardiac glycosides in colon cancer cell...
2009-11-01 [J. Nat. Prod. 72 , 1969-74, (2009)] |
|
Role of endogenous cardiac glycosides in the spontaneously h...
1996-01-01 [Am. J. Hypertens. 9(1) , 81-5, (1996)] |
|
Specific anti-digoxin Fab fragments: an available antidote f...
1990-05-01 [Hum. Exp. Toxicol. 9(3) , 191-3, (1990)] |
|
Proscillaridin A immunoreactivity: its purification, transpo...
1998-01-01 [Clin. Exp. Hypertens. 20(5-6) , 593-9, (1998)] |