Synthesis of allo- and epi-inositol via the NHC-catalyzed carbocyclization of carbohydrate-derived dialdehydes.
Kieran P Stockton, Ben W Greatrex, Dennis K Taylor
文献索引:J. Org. Chem. 79(11) , 5088-96, (2014)
全文:HTML全文
摘要
A synthesis of carbocyclic sugars from carbohydrate-derived dialdehydes using organocatalysis has been developed. Sorbitol, mannitol, and galactitol were converted via 1,6-tritylation, perbenzylation or permethylation, detritylation, and Swern oxidation into 2,3,4,5-tetra-O-alkyl-dialdoses that were cyclized via the benzoin reaction promoted by a triazolium carbene. Manno- and galacto-configured dialdehydes gave predominantly single inosose stereoisomers in up to 75% yield if the mixture was acetylated prior to isolation while the gluco-dialdehyde afforded a mixture of three stereoisomers in 61% overall yield. The inososes were stereospecifically reduced using sodium borohydride and then deprotected to give allo- and epi-inositol in good yield that confirmed the structural and stereochemical assignments.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
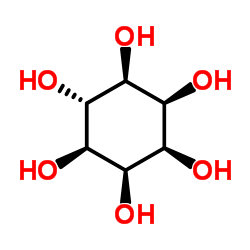 |
表肌醇
CAS:488-58-4 |
C6H12O6 |
|
Synthesis of phosphatidylinositols having various inositol s...
2010-04-01 [J. Biosci. Bioeng. 109(4) , 337-40, (2010)] |
|
Cyclohexanehexol inhibitors of Abeta aggregation prevent and...
2006-07-01 [Nat. Med. 12(7) , 801-8, (2006)] |
|
Epi-inositol regulates expression of the yeast INO1 gene enc...
2002-01-01 [Mol. Psychiatry 7(2) , 174-80, (2002)] |
|
Inositol stereoisomers stabilize an oligomeric aggregate of ...
2000-06-16 [J. Biol. Chem. 275(24) , 18495-502, (2000)] |
|
Topological and functional characterization of an insect gus...
2011-01-01 [PLoS ONE 6(8) , e24111, (2011)] |