Nuclear magnetic resonance studies of 6-fluorotryptophan-substituted rat cellular retinol-binding protein II produced in Escherichia coli. Analysis of the apoprotein and the holoprotein containing bound all-trans-retinol and all-trans-retinal.
E Li, S J Quian, L Nader, N C Yang, A d'Avignon, J C Sacchettini, J I Gordon
文献索引:J. Biol. Chem. 264(29) , 17041-8, (1989)
全文:HTML全文
摘要
Rat cellular retinol-binding protein II (CRBP II) is a 15.6-kDa intestinal protein which binds all-trans-retinol and all-trans-retinal but not all-trans-retinoic acid. We have previously analyzed the interaction of Escherichia coli-derived rat apoCRBP II with several retinoids using fluorescence spectroscopic techniques. Interpretation of these experiments is complicated, because the protein has 4 tryptophan residues. To further investigate ligand-protein interactions, we have utilized 19F nuclear magnetic resonance (NMR) spectroscopy of CRBP II labeled at its 4 tryptophan residues with 6-fluorotryptophan. Efficient incorporation of 6-fluorotryptophan (93%) was achieved by growing a tryptophan auxotroph of E. coli harboring a prokaryotic expression vector with a full-length rat CRBP II cDNA on defined medium supplemented with the analog. Comparison of the 19F NMR spectra of 6-fluorotryptophan-substituted CRBP II with and without bound all-trans-retinol revealed that resonances corresponding to 2 tryptophan residues (designated WA and WB) undergo large downfield changes in chemical shifts (2.0 and 0.5 ppm, respectively) associated with ligand binding. In contrast, 19F resonances corresponding to two other tryptophan residues (WC and WD) undergo only minor perturbations in chemical shifts. The 19F NMR spectra of 6-fluorotryptophan-substituted CRBP II complexed with all-trans-retinal and all-trans-retinol were very similar, suggesting that the interactions of these two ligands with the protein are similar. Molecular model building, based on the crystalline structures of two homologous proteins was used to predict the positions of the 4 tryptophan residues of CRBP II and to make tentative resonance assignments. The fact that ligand binding produced residue-specific changes in the chemical shifts of resonances in CRBP II suggests that NMR analysis of isotopically labeled retinoid-binding proteins expressed in E. coli will provide an alternate, albeit it complementary, approach to fluorescence spectroscopy for examining the structural consequences of their association with ligand.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
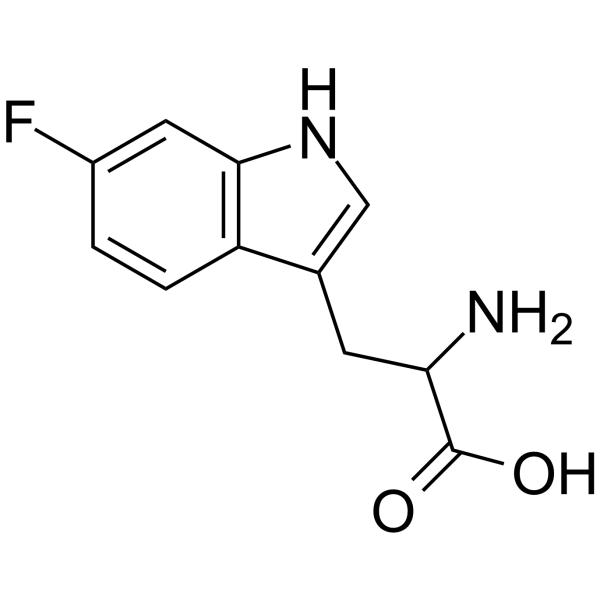 |
6-氟-DL-色氨酸
CAS:7730-20-3 |
C11H11FN2O2 |
|
Substrate promiscuity of the cyclic dipeptide prenyltransfer...
2009-01-01 [J. Nat. Prod. 72 , 44-52, (2009)] |
|
Cloning of the trp gene cluster from a tryptophan-hyperprodu...
1993-03-01 [Appl. Environ. Microbiol. 59(3) , 791-9, (1993)] |
|
Atomic mutations at the single tryptophan residue of human r...
1999-08-17 [Biochemistry 38(33) , 10649-59, (1999)] |
|
Incorporation of tryptophan analogues into staphylococcal nu...
1998-06-23 [Biochemistry 37(25) , 8938-46, (1998)] |
|
Phosphorescence and optically detected magnetic resonance ch...
1998-06-23 [Biochemistry 37(25) , 8954-64, (1998)] |