Reactions of dihydrodiol epoxides of 5-methylchrysene and 5, 6-dimethylchrysene with DNA and deoxyribonucleotides.
J Szeliga, S Amin, F J Zhang, R G Harvey
文献索引:Chem. Res. Toxicol. 12(4) , 347-52, (1999)
全文:HTML全文
摘要
Both syn and anti dihydrodiol epoxides from 5-methylchrysene (5-MCDE) and 5,6-dimethylchrysene (5,6-DMCDE) were reacted under the same conditions with native DNA, denatured DNA, and purine deoxyribonucleotides, and the products were quantified. The extents of reaction with the deoxyribonucleotides were consistently greater for 5,6-DMCDE than for 5-MCDE. The yield of adducts in the reaction with DNA ranged from being a few-fold to 50-fold greater than those found in the corresponding deoxyribonucleotide reactions for both 5-MCDE and 5,6-DMCDE. The DNA-dependent enhancement of product yield was greater for 5-MCDE than for 5,6-DMCDE with a few exceptions among cis and trans deoxyadenosine adducts. The most substantial differences in DNA-dependent enhancement were found for deoxyguanosine adducts; thus, steric hindrance between the 6-methyl group in the 5,6-DMCDE and the minor groove in the DNA double helix may account for the greater DNA-dependent enhancement found in the 5-MCDE reactions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
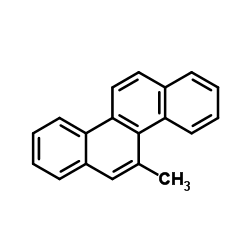 |
5-甲基-1,2-苯并菲
CAS:3697-24-3 |
C19H14 |
|
QSPR modeling of octanol/water partition coefficient for vit...
2008-04-01 [Eur. J. Med. Chem. 43 , 714-40, (2008)] |
|
Polycyclic aromatic hydrocarbons: 15 Listings - benz[a]anthr...
2011-01-01 [Rep. Carcinog. 12 , 353-61, (2011)] |
|
TET1 controls CNS 5-methylcytosine hydroxylation, active DNA...
2013-09-18 [Neuron 79(6) , 1086-93, (2013)] |
|
Analysis of carcinogenic polycyclic aromatic hydrocarbons in...
2011-09-30 [Anal. Chim. Acta 702(2) , 218-24, (2011)] |
|
Tumor multiplicity, DNA adducts and K-ras mutation pattern o...
1994-11-01 [Carcinogenesis 15(11) , 2613-8, (1994)] |