Na+-pyrophosphatase: a novel primary sodium pump.
Anssi M Malinen, Georgiy A Belogurov, Alexander A Baykov, Reijo Lahti
文献索引:Biochemistry 46(30) , 8872-8, (2007)
全文:HTML全文
摘要
Membrane-bound pyrophosphatase (PPase) is commonly believed to couple pyrophosphate (PPi) hydrolysis to H+ transport across the membrane. Here, we demonstrate that two newly isolated bacterial membrane PPases from the mesophile Methanosarcina mazei (Mm-PPase) and the moderate thermophile Moorella thermoacetica and a previously described PPase from the hyperthermophilic bacterium Thermotoga maritima catalyze Na+ rather than H+ transport into Escherichia coli inner membrane vesicles (IMV). When assayed in uncoupled IMV, the three PPases exhibit an absolute requirement for Na+ but display the highest hydrolyzing activity in the presence of both Na+ and K+. Steady-state kinetic analysis of PPi hydrolysis by Mm-PPase revealed two Na+ binding sites. One of these sites can also bind K+, resulting in a 10-fold increase in the affinity of the other site for Na+ and a 2-fold increase in maximal velocity. PPi-driven 22Na+ transport into IMV containing Mm-PPase was unaffected by the protonophore carbonyl cyanide m-chlorophenylhydrazone, inhibited by the Na+ ionophore monensin, and activated by the K+ ionophore valinomycin. The Na+ transport was accompanied by the generation of a positive inside membrane potential as reported by Oxonol VI. These findings define Na+-dependent PPases as electrogenic Na+ pumps. Phylogenetic analysis suggests that ancient gene duplication preceded the split of Na+- and H+-PPases.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
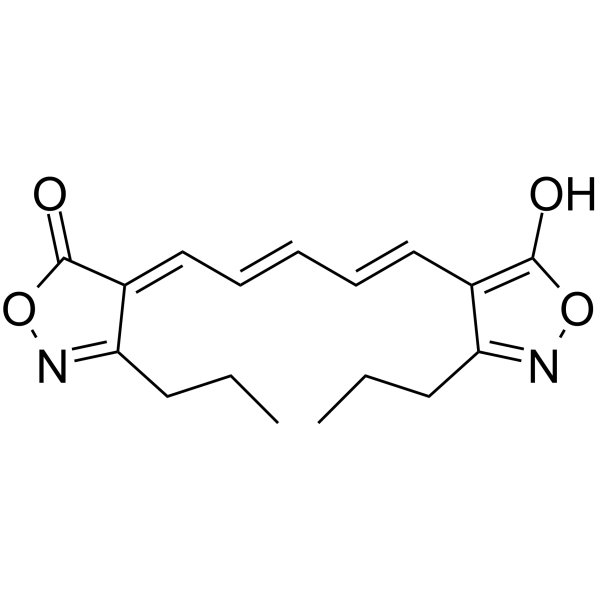 |
1,5-双(5-氧-3-丙基异唑-4-基)五甲川氧醇
CAS:64724-75-0 |
C17H20N2O4 |
|
Conserved amino acid residues of the NuoD segment important ...
2015-01-27 [Biochemistry 54(3) , 753-64, (2015)] |
|
ATP-dependent spectral response of oxonol VI in an ATP-Pi ex...
1984-08-31 [Biochim. Biophys. Acta 766(2) , 375-85, (1984)] |
|
Effects of Cd2+ on ATP-driven membrane potential in beef hea...
1984-01-01 [Membr. Biochem. 5(3) , 225-41, (1984)] |
|
A stopped-flow kinetic study of the interaction of potential...
1989-11-01 [Biophys. Chem. 34(3) , 225-37, (1989)] |
|
Electrophysiological study with oxonol VI of passive NO3- tr...
1999-01-01 [Biophys. J. 76(1 Pt 1) , 360-73, (1999)] |