omega-Hydroxylation of Z9-octadecenoic, Z9,10-epoxystearic and 9,10-dihydroxystearic acids by microsomal cytochrome P450 systems from Vicia sativa.
F Pinot, J P Salaün, H Bosch, A Lesot, C Mioskowski, F Durst
文献索引:Biochem. Biophys. Res. Commun. 184(1) , 183-93, (1992)
全文:HTML全文
摘要
A microsomal fraction from etiolated Vicia sativa seedlings incubated aerobically with [1-14C]oleic acid (Z9-octadecenoic acid) or [1-14C]9,10-epoxystearic acid or [1-14C]9,10-dihydroxystearic acid catalyzed the NADPH-dependent formation of hydroxylated metabolites. The chemical structure of compounds formed from oleic, 9,10-epoxystearic or 9,10-dihydroxystearic acids was established by gas chromatography/mass spectra analysis to be 18-hydroxyoleic acid, 18-hydroxy-9,10-epoxystearic acid and 9,10,18-trihydroxystearic acid, respectively. The reactions required O2 and NADPH and were inhibited by carbon monoxide. As expected for monooxygenase reactions involving cytochrome P450, inhibition could be partially reversed by light and all three reactions were inhibited by antibodies raised against NADPH-cytochrome P450 reductase from Jerusalem artichoke. The omega-hydroxylation of the three substrates was enhanced in microsomes from clofibrate induced seedlings.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
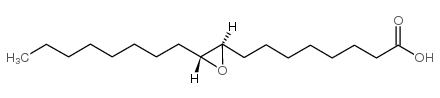 |
反式-9,10-环氧十八烷酸
CAS:2443-39-2 |
C18H34O3 |
|
GC-MS/MS and LC-MS/MS studies on unlabelled and deuterium-la...
2014-08-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 964 , 172-9, (2014)] |
|
Cloning, functional expression, and characterization of CYP7...
2005-10-28 [J. Biol. Chem. 280(43) , 35881-9, (2005)] |
|
Determination of 9,10-epoxystearate ester in intravenous sol...
1984-07-01 [Analyst 109(7) , 967-8, (1984)] |
|
Gas chromatography-mass spectrometry of cis-9,10-epoxyoctade...
2004-05-25 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 804(2) , 403-12, (2004)] |
|
Analysis of the seed oil of Heisteria silvanii (Olacaceae)--...
1997-11-01 [Lipids 32(11) , 1189-200, (1997)] |