Formation of thymidine adducts and cross-linking potential of 2-bromoacrolein, a reactive metabolite of tris(2,3-dibromopropyl)phosphate.
G J van Beerendonk, M J Nivard, E W Vogel, S D Nelson, J H Meerman
文献索引:Mutagenesis 7(1) , 19-24, (1992)
全文:HTML全文
摘要
DNA-adduct formation by the mutagen 2-bromoacrolein (2BA) with DNA was studied. [3-3H]2BA was reacted with single-stranded (ss) DNA or double-stranded (ds) DNA and subsequently incubated with methoxylamine to convert an unstable 2BA:thymidine adduct (Meerman et al., Cancer Res., 49, 6174-6179, 1989) to a stable product. This product was identified in the study as 3-(2"-hydroxy-3"-methoximpropyl)thymidine (HYMETH) by LC-MS. After extensive purification and enzymatic hydrolysis of modified ssDNA and dsDNA, approximately 5% of the covalently bound activity coeluted with added HYMETH standard in a reverse phase HPLC system. Because the unstable 2BA:thymidine adduct may have the potential to form cross-links, we investigated the reaction of this adduct with various nucleophiles in vitro. A reaction occurred between the adduct and cysteine, but not with lysine or deoxynucleosides. Reaction of 2BA with ssDNA in the presence of [3H]glutathione also resulted in the binding of radiolabelled GSH to DNA. These results indicate that the reactive aldehyde group of the adduct can react with thiol groups in proteins to form protein-DNA cross-links. Further, the possibility that tris- and bis-(2,3-dibromopropyl)phosphate (Tris-BP and Bis-BP) form such cross-links was examined in vivo in Drosophila. The results indicate that Tris-BP is a cross-linking agent, whereas Bis-BP is not. Inasmuch as Tris-BP is known to be metabolized rapidly to 2BA and Bis-BP, whereas Bis-BP forms 2BA only very slowly, suggests that in Drosophila DNA adducts are formed that cross-link proteins.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
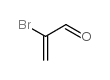 |
2-溴丙烯醛
CAS:14925-39-4 |
C3H3BrO |
|
Metabolism and genotoxicity of the halogenated alkyl compoun...
1994-12-01 [Hum. Exp. Toxicol. 13(12) , 861-5, (1994)] |
|
Genotoxicity of 2-halosubstituted enals and 2-chloroacryloni...
1994-10-01 [Mutat. Res. 322(4) , 321-8, (1994)] |
|
Formation of thymidine, cyclic deoxyguanosine and cyclic deo...
1994-01-01 [IARC Sci. Publ. (125) , 449-52, (1994)] |
|
Formation of cyclic 1,N2-propanodeoxyguanosine and thymidine...
1989-11-15 [Cancer Res. 49(22) , 6174-9, (1989)] |
|
Roles of C-H...O=S and pi-stacking interactions in the 2-bro...
2005-07-08 [J. Org. Chem. 70(14) , 5487-93, (2005)] |