Site-specific incorporation of arginine analogs into proteins using arginyl-tRNA synthetase.
Akiya Akahoshi, Yoshitaka Suzue, Mizuki Kitamatsu, Masahiko Sisido, Takashi Ohtsuki
文献索引:Biochem. Biophys. Res. Commun. 414(3) , 625-30, (2011)
全文:HTML全文
摘要
Arginine analogs were incorporated site-specifically into proteins using an in vitro translation system. In this system, mRNAs containing a CGGG codon were translated by an aminoacyl-tRNA(CCCG), which was charged with arginine analogs using yeast arginyl-tRNA synthetase. N(G)-monomethyl-L-arginine, L-citrulline and L-homoarginine were incorporated successfully into proteins using this method. The influence of arginine monomethylation in histone H3 on the acetylation of lysine residues by histone acetyltransferase hGCN5 was investigated, and the results demonstrated that K9 acetylation was suppressed by the methylation of R8 and R17 but not by R26 methylation. K18 acetylation was not affected by the methylation of R8, R17 and R26. This site-specific modification strategy provides a way to explore the roles of post-translational modifications in the absence of heterogeneity due to other modifications.Copyright © 2011 Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
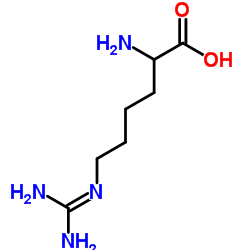 |
L-高精氨酸盐酸盐
CAS:1483-01-8 |
C7H17ClN4O2 |
|
Measurement of homoarginine in human and mouse plasma by LC-...
2015-09-01 [Amino Acids 47 , 2015-22, (2015)] |
|
Elevated Levels of Asymmetric Dimethylarginine (ADMA) in the...
2015-01-01 [PLoS ONE 10 , e0135498, (2015)] |
|
Influence of L-homoarginine as an analogue of L-arginine on ...
2012-09-01 [Biotechnol. Prog. 31 , 808-14, (2015)] |
|
Asymmetric Synthesis of Conformationally Restricted L-Argini...
2001-01-01 [J. Org. Chem. 64 , 3467-3475, (1999)] |
|
Amino acids as modulators of endothelium-derived nitric oxid...
[Am. J. Physiol. Renal Physiol. 291 , F297-304, (2006)] |