Total synthesis and biological evaluation of clavaminol-G and its analogs.
T Vijai Kumar Reddy, B L A Prabhavathi Devi, R B N Prasad, P Sujitha, C Ganesh Kumar
文献索引:Eur. J. Med. Chem. 67 , 384-9, (2013)
全文:HTML全文
摘要
The first total synthesis of clavaminol-G (1) and 1-aminoundecan-2-ol (2) has been achieved from 10-undecenoic acid using epoxidation, regioselective azidolysis and in situ detosylation and reduction reactions as key steps. The methodology is extended for the synthesis of 1-aminoundecan-2-ol derivatives; namely, methyl 11-amino-10-hydroxyundecanoate (3), 11-amino-10-hydroxyundecanoic acid (4) and 11-aminoundecan-1,10-diol (5). Among these, 1-aminoundecan-2-ol (2) exhibited good antimicrobial activity and promising cytotoxicity towards HeLa, MDA-MB-231, MCF-7 and A549 cell lines with IC50 values of 4.36, 4.02, 3.88 and 6.78 μM, respectively. Compound 3 exhibited good activity against HeLa cells (IC50 = 3.59 μM), while compound 5 showed moderate activity towards HeLa and A549 cell lines. Clavaminol G (1) and compound 4 showed no activity towards all the cell lines. Copyright © 2013 Elsevier Masson SAS. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
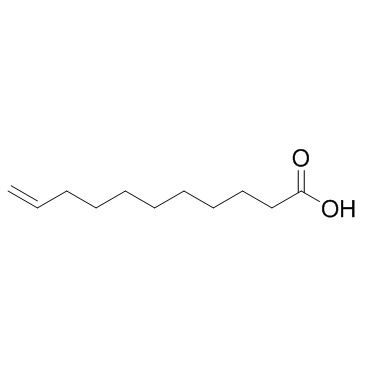 |
十一烯酸
CAS:112-38-9 |
C11H20O2 |
|
Mechanism of erosion of nanostructured porous silicon drug c...
2015-01-01 [Nat. Commun. 6 , 6208, (2015)] |
|
Development of L-lactate dehydrogenase biosensor based on po...
2015-12-15 [Biosens. Bioelectron. 74 , 637-43, (2015)] |
|
Trifunctional Polymeric Nanocomposites Incorporated with Fe₃...
2015-11-11 [ACS Appl. Mater. Interfaces 7 , 24523-32, (2015)] |
|
Potent in vitro antifungal activities of naturally occurring...
2008-07-01 [Antimicrob. Agents Chemother. 52 , 2442-8, (2008)] |
|
Tailoring of interfacial mechanical shear strength by surfac...
2015-05-26 [ACS Nano 9 , 5143-53, (2015)] |