Lowering of 5-nitroimidazole's mutagenicity: towards optimal antiparasitic pharmacophore.
Maxime D Crozet, Céline Botta, Monique Gasquet, Christophe Curti, Vincent Rémusat, Sébastien Hutter, Olivier Chapelle, Nadine Azas, Michel De Méo, Patrice Vanelle
文献索引:Eur. J. Med. Chem. 44(2) , 653-9, (2009)
全文:HTML全文
摘要
To improve the antiparasitic pharmacophore, 20 5-nitroimidazoles bearing an arylsulfonylmethyl group were prepared from commercial imidazoles. The antiparasitic activity of these molecules was assessed against Trichomonas vaginalis, the in vitro cytotoxicity was evaluated on human monocytes and the mutagenicity was determined by the Salmonella mutagenicity assay. All IC(50) on T. vaginalis were below the one of metronidazole. The determination of the specificity indexes (SIs), defined as the ratios of the cytotoxic activity and the antitrichomonas activity, indicated that 11 derivatives had a SI over the one of metronidazole. Molecules, bearing an additional methyl group on the 2-position, showed a lower mutagenicity than metronidazole. Moreover, three derivatives were characterized by a low mutagenicity and an efficient antitrichomonas activity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
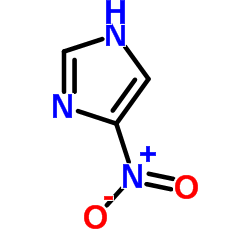 |
4-硝基咪唑
CAS:3034-38-6 |
C3H3N3O2 |
|
Binding of imidazole, 1-methylimidazole and 4-nitroimidazole...
2015-08-01 [Biochim. Biophys. Acta 1854 , 869-81, (2015)] |
|
Apolar distal pocket mutants of yeast cytochrome c peroxidas...
2015-08-01 [Biochim. Biophys. Acta 1854 , 919-29, (2015)] |
|
Analysis of four 5-nitroimidazoles and their corresponding h...
2006-03-22 [J. Agric. Food Chem. 54(6) , 2018-26, (2006)] |
|
Synthesis of some N-substituted nitroimidazole derivatives a...
2009-02-01 [Eur. J. Med. Chem. 44 , 645-52, (2009)] |
|
New synthesis and antiparasitic activity of model 5-aryl-1-m...
2009-01-01 [Molecules 14(8) , 2758-67, (2009)] |