A Drug Safety Rating System Based on Postmarketing Costs Associated with Adverse Events and Patient Outcomes.
Keith B Hoffman, Mo Dimbil, Robert F Kyle, Nicholas P Tatonetti, Colin B Erdman, Andrea Demakas, Dingguo Chen, Brian M Overstreet
文献索引:J. Manag. Care. Spec. Pharm. 21 , 1134-43, (2015)
全文:HTML全文
摘要
Given the multiple limitations associated with relatively homogeneous preapproval clinical trials, inadequate data disclosures, slow reaction times from regulatory bodies, and deep-rooted bias against disclosing and publishing negative results, there is an acute need for the development of analytics that reflect drug safety in heterogeneous, real-world populations.To develop a drug safety statistic that estimates downstream medical costs associated with serious adverse events (AEs) and unfavorable patient outcomes associated with the use of 706 FDA-approved drugs.All primary suspect case reports for each drug were collected from the FDA's Adverse Event Reporting System database (FAERS) from 2010-2014. The Medical Dictionary for Regulatory Activities (MedDRA) was used to code serious AEs and outcomes, which were tallied for each case report. Medical costs associated with AEs and poor patient outcomes were derived from Agency for Healthcare Research and Quality (AHRQ) survey data, and their corresponding ICD-9-CM codes were mapped to MedDRA terms. Nonserious AEs and outcomes were not included. For each case report, either the highest AE cost or, if no eligible AE was listed, the highest outcome cost was used. All costed cases were aggregated for each drug and divided by the number of patients exposed to obtain a downstream estimated direct medical cost burden per exposure. Each drug was assigned a corresponding 1-100 point total.The 706 drugs showed an exponential distribution of downstream costs, and the data were transformed using the natural log to approximate a normal distribution. The minimum score was 8.29, and the maximum score was 99.25, with a mean of 44.32. Drugs with the highest individual scores tended to be kinase inhibitors, thalidomide analogs, and endothelin receptor antagonists. When scores were analyzed across Established Pharmacologic Class (EPC), the kinase inhibitor and endothelin receptor antagonist classes had the highest total. However, other EPCs with median scores of 75 and above included hepatitis C virus NS3/4A protease inhibitor, recombinant human interferon beta, vascular endothelial growth factor-directed antibody, and tumor necrosis factor blocker. When Anatomical Therapeutic Chemical classifications were analyzed, antineoplastic drugs were outliers with approximately 80% of their individual scores 60 and above, while approximately 20%-30% of blood and anti-infective drugs had scores of 60 and above. Within-drug class results served to differentiate similar drugs. For example, 6 serotonin reuptake inhibitors had a score range of 35 to 53.This scoring system is based on estimated direct medical costs associated with postmarketing AEs and poor patient outcomes and thereby helps fill a large information gap regarding drug safety in real-world patient populations.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
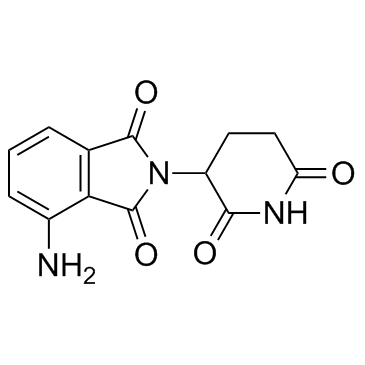 |
泊马度胺
CAS:19171-19-8 |
C13H11N3O4 |
|
Rational combination treatment with histone deacetylase inhi...
2015-01-01 [Blood Cancer J. 5 , e312, (2015)] |
|
Pomalidomide: evaluation of cytochrome P450 and transporter-...
2015-02-01 [J. Clin. Pharmacol. 55(2) , 168-78, (2015)] |
|
A validated UPLC-MS/MS assay using negative ionization mode ...
2015-03-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 983-984 , 76-82, (2015)] |
|
Targeting of BMI-1 with PTC-209 shows potent anti-myeloma ac...
2016-01-01 [J. Hematol. Oncol. 9 , 17, (2016)] |
|
Immunomodulatory drugs improve the immune environment for de...
2014-10-01 [Cancer Immunol. Immunother. 63(10) , 1023-36, (2014)] |