Effect of concomitant hormone replacement therapy containing estradiol and levonorgestrel on the pharmacokinetics of selegiline.
Sanna Palovaara, Markku Anttila, Leena Nyman, Kari Laine
文献索引:Eur. J. Clin. Pharmacol. 58(4) , 259-63, (2002)
全文:HTML全文
摘要
The aim of this study was to investigate the effect of hormone-replacement therapy (HRT) on the pharmacokinetics of the selective monoamine oxidase B inhibitor selegiline and its primary metabolites desmethylselegiline and l-metamphetamine.In this randomised, double-blind, cross-over trial, 12 healthy female subjects received once daily for 10 days either HRT containing 2 mg estradiol valerate and 250 microg levonorgestrel or matched placebo. On day 10, they took a single 10-mg oral dose of selegiline. The serum concentrations of selegiline, desmethylselegiline and metamphetamine were measured for 32 h.There was a 59% difference ( P=0.14) in the area under the serum concentration-time curve (AUC) of selegiline during the HRT compared with the placebo phase, but only a little or no concomitant reduction in the AUC of desmethylselegiline (-7%, P=0.071) or metamphetamine (2%, P=0.614) was observed. Maximum plasma concentration (C(max)) of selegiline was not changed, but a small, statistically significant, reduction in the C(max) of desmethylselegiline (-17%, P=0.03) was seen during the HRT phase. The C(max) of methamphetamine was slightly but not significantly reduced (-5%, P=0.06). The unchanged AUC ratios of desmethylselegiline/selegiline and metamphetamine/selegiline indicate that the primary metabolism of selegiline was not affected by HRT. All study treatments were well tolerated.Unlike oral contraceptives, HRT is not likely to have clinically significant pharmacokinetic interaction with selegiline.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
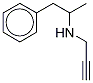 |
去甲塞利吉林
CAS:18913-84-3 |
C12H15N |
|
Simultaneous determination of selegiline and desmethylselegi...
2006-12-05 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 844(2) , 283-91, (2006)] |
|
Modulation of neuroendocrine--immune signaling by L-deprenyl...
2002-04-30 [Mech. Ageing Dev. 123(8) , 1065-79, (2002)] |
|
Pharmacokinetic studies of (-)-deprenyl and some of its meta...
2007-01-01 [J. Neural Transm. Suppl. (72) , 165-73, (2007)] |
|
P450 phenotyping of the metabolism of selegiline to desmethy...
2007-04-01 [Drug Metab. Pharmacokinet. 22(2) , 78-87, (2007)] |
|
Multiple-dose pharmacokinetics of selegiline and desmethylse...
2000-01-01 [Clin. Neuropharmacol. 23(1) , 22-7, (2000)] |