Fluorescence of intramolecular and intermolecular interactions of aminonaphthyl-sulfonate with nucleotides.
J Fick, R Lawaczeck, F W Schneider
文献索引:Eur. J. Biochem. 126(2) , 367-72, (1982)
全文:HTML全文
摘要
Fluorescence studies of the intramolecular and intermolecular interactions between aminonaphthylsulfonate and nucleotides of uracil or adenine are described. The fluorescence originates solely from the naphthyl moiety and is intramolecularly quenched by the base, uracil being more effective than adenine. The enzymatic splitting of the molecule into a nucleoside monophosphate and the pyrophosphate product of the aminonaphthylsulfonate removes the intramolecular quenching and, especially in the case of uracil, a drastic increase of the fluorescence intensity results. The intact molecule exists predominantly in the folded form except in cases where electrostatic repulsion exceeds the stacking attraction. This is borne out by the pH dependence and the existence of a pronounced solvent-isotope effect of the fluorescence quantum yield for the uracil derivative at basic pH. At pH values above the pK of the enol proton of the uracil base the fluorescent properties of the intact and phosphodiesterase-digested molecules are very similar. The intermolecular interactions between 1-aminonaphthalene-5-sulfonate with AMP and UMP can be explained on the basis of dynamic quenching (collisional quenching) without any significant participation of ground-state complexes (static quenching). The interaction of the pyrophosphate adduct of 1-aminonaphthalene-5-sulfonate with UMP can best be explained by invoking two interacting nucleotide species: the free nucleotide and a sodium-nucleotide complex.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
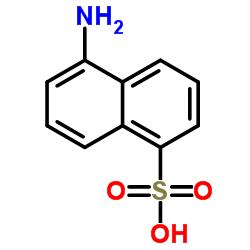 |
5-氨基-1-萘磺酸
CAS:84-89-9 |
C10H9NO3S |
|
Identification of a novel ligand binding motif in the transt...
2010-01-01 [Bioorg. Med. Chem. 18 , 100-10, (2010)] |
|
[An interesting peroxidase staining.--utilization of blended...
1981-06-01 [Rinsho Byori. 29(6) , 613-6, (1981)] |
|
Evidence for a ppGpp-binding site on Escherichia coli RNA po...
1995-01-01 [Mol. Microbiol. 15(2) , 255-65, (1995)] |
|
Controlling {beta}-amyloid oligomerization by the use of nap...
2005-10-14 [J. Biol. Chem. 280(41) , 34747-54, (2005)] |
|
Nucleotide modification at the gamma-phosphate leads to the ...
2005-01-01 [Nucleic Acids Res. 33(15) , 4865-73, (2005)] |