Binding of isofraxidin to bovine serum albumin.
Jiaqin Liu, Jianniao Tian, Zhide Hu, Xingguo Chen
文献索引:Biopolymers 73(4) , 443-50, (2004)
全文:HTML全文
摘要
The binding of isofraxidin to bovine serum albumin (BSA) was studied under physiological conditions with BSA concentration of 1.5 x 10(-6) mol x L(-1) and drug concentration in the range of 1.67 x 10(-6) mol x L(-1) to 2.0 x 10(-5) mol x L(-1). Fluorescence quenching spectra in combination with uv absorption spectroscopy, Fourier transform infrared (FTIR) spectroscopy, and CD spectroscopy was used to determine the drug-binding mode, binding constant, and the protein structure changes in the presence of isofraxidin in aqueous solution. The linearity of Scatchard plot indicates that isofraxidin binds to a single class of binding sites on BSA and the values given for the binding constants agree very closely with those obtained by the modified Stern-Volmer equation. The thermodynamic parameters, enthalpy change (DeltaH) and entropy change (DeltaS), were calculated to be -17.63 kJ x mol(-1) and 51.38 J x mol(-1) x K(-1) according to the van't Hoff equation, which indicated that hydrophobic interaction played a main role in the binding of isofraxidin to BSA.Copyright 2004 Wiley Periodicals, Inc.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
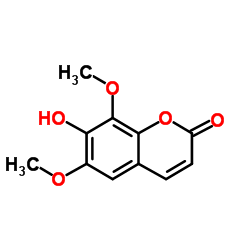 |
异秦皮啶
CAS:486-21-5 |
C11H10O5 |
|
Determination of isofraxidin and astilbin by HPLC in rat pla...
2013-01-01 [Pak. J. Pharm. Sci. 26(1) , 1-6, (2013)] |
|
Active components from Siberian ginseng (Eleutherococcus sen...
2011-07-01 [J. Nat. Med. 65(3-4) , 417-23, (2011)] |
|
Simultaneous determination of protocatechuic acid, syringin,...
2006-03-01 [Biol. Pharm. Bull. 29(3) , 532-4, (2006)] |
|
A rapid and sensitive UPLC-ESI MS method for analysis of iso...
2007-12-01 [J. Sep. Sci. 30(18) , 3202-6, (2007)] |
|
Isofraxidin, a coumarin component from Acanthopanax senticos...
2010-01-01 [Biol. Pharm. Bull. 33(10) , 1716-22, (2010)] |