ortho-Phenylene oligomers with terminal push-pull substitution.
Jian He, Sanyo M Mathew, Sarah D Cornett, Stephan C Grundy, C Scott Hartley
文献索引:Org. Biomol. Chem. 17th ed., 10 , 3398-3405, (2012)
全文:HTML全文
摘要
ortho-Phenylenes are an emerging class of helical oligomers and polymers. We have synthesized a series of push-pull-substituted o-phenylene oligomers (dimethylamino/nitro) up to the octamer. Conformational analysis of the hexamer using a combination of low-temperature NMR spectroscopy and ab initio predictions of (1)H NMR chemical shifts indicates that, like other o-phenylenes, they exist as compact helices in solution. However, the substituents are found to have a significant effect on their conformational behavior: the nitro-functionalized terminus is 3-fold more likely to twist out of the helix. Protonation of the dimethylamino group favors the helical conformer. UV/vis spectroscopy indicates that the direct charge-transfer interaction between the push-pull substituents attenuates quickly compared to other conjugated systems, with no significant charge-transfer band for oligomers longer than the trimer. On protonation of the dimethylamino group, significant bathochromic shifts with increasing oligomer length are observed: the effective conjugation length is 9 repeat units, more than twice that of the parent oligomer. This behavior may be rationalized through examination of the frontier molecular orbitals of these compounds, which exhibit greater delocalization after protonation, as shown by DFT calculations.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
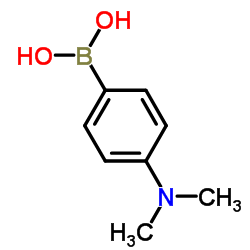 |
4-(二甲基氨基)苯硼酸
CAS:28611-39-4 |
C8H12BNO2 |
|
Synthesis and photophysical investigation of a series of pus...
2012-04-20 [J. Org. Chem. 8th ed., 77 , 4087-4096, (2012)] |
|
Nickel-catalyzed Suzuki-Miyaura reaction of aryl fluorides.
2011-12-07 [J. Am. Chem. Soc. 48th ed., 133 , 19505-19511, (2011)] |
|
Dramatic Effect of the Gelling Cation on the Catalytic Perfo...
[Chem. Mater. 8th ed., 24 , 1505-1510, (2012)] |
|
Organic dyes incorporating the cyclopentadithiophene moiety ...
[Dyes and Pigments 3rd ed., 92 , 1292-1299, (2012)] |
|
Rapid access to 4-substituted-pyrones and 2(5H)-furanones vi...
[Tetrahedron 38th ed., 67 , 7258-7262, (2011)] |