Mutations affecting a regulated, membrane-associated esterase in Salmonella typhimurium LT2.
P Collin-Osdoby, C G Miller
文献索引:Mol. Gen. Genet. 243(6) , 674-80, (1994)
全文:HTML全文
摘要
Mutations at the apeA locus in Salmonella typhimurium lead to loss of a soluble enzyme ("protease I") that hydrolyzes the chromogenic endoprotease substrate N-acetyl phenylalanine beta-naphthyl ester. We have isolated pseudorevertants of S. typhimurium apeA mutations that have regained the ability to hydrolyze this compound. These pseudorevertants contain mutations (apeR) that lead to overproduction of a membrane-bound esterase different from protease I. The apeR locus is phage P1 cotransducible with ilvC (83 map units) and is unlinked to apeA. Mutations at still another locus, apeE, lead to loss of the membrane-associated esterase. The apeE locus is P1 cotransducible with purE (12 map units). In an apeE-lacZ operon fusion strain, an apeR mutation increases the level of beta-galactosidase approximately 60-fold. We propose that apeR encodes a repressor of apeE. The evidence available suggests that the ApeE protein is not a protease.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
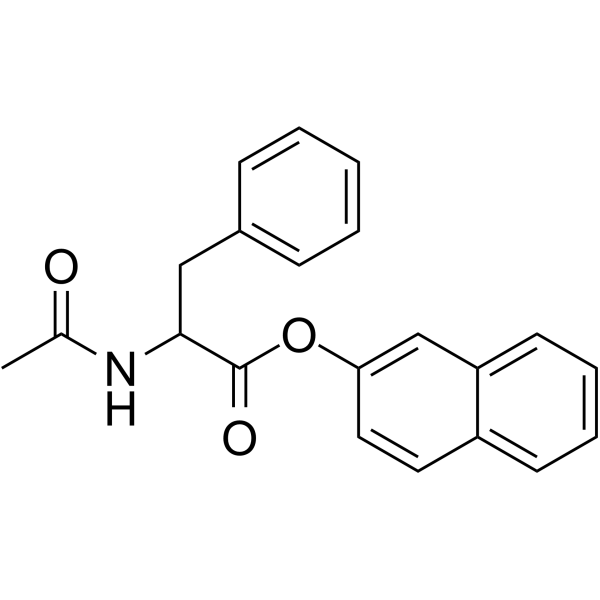 |
N-乙酰-DL-苯丙氨酸2-萘基酯
CAS:20874-31-1 |
C21H19NO3 |
|
A simple method to determine trypsin and chymotrypsin inhibi...
2004-06-30 [J. Biochem. Biophys. Methods 59 , 241-251, (2004)] |
|
An association between Schistosoma mansoni worms and an enzy...
2008-04-01 [Parasitology 135 , 467-472, (2008)] |
|
Involvement of proteases in the action of IFN-gamma on WISH ...
2002-08-01 [J. Interferon Cytokine Res. 22(8) , 847-52, (2002)] |
|
Assay for enzyme inhibition: detection of natural inhibitors...
1987-05-01 [Anal. Biochem. 162 , 345, (1987)] |
|
Electrophoretic separation of proteolytic enzymes in pancrea...
1997-08-01 [Int. J. Pancreatol. 22 , 39-43, (1997)] |