[Rufinamide. A review of its pharmacokinetic and pharmacodynamic properties].
J L Herranz
文献索引:Rev. Neurol. 47(7) , 369-73, (2008)
全文:HTML全文
摘要
To review the most important research published on the pharmacokinetic and pharmacodynamic properties of rufinamide (RFM), together with the outcomes of the clinical trials conducted to date with this new antiepileptic drug.RFM is a triazole derivative with no structural relation to the other antiepileptic drugs, and the effectiveness and safety profiles shown in laboratory animals suggest that it could be effective in the treatment of partial and generalised seizures. In fact, in double-blind trials RFM has proved to be effective and well tolerated in adults with intractable partial seizures and in patients between 4 and 30 years of age with Lennox-Gastaut syndrome.Based on the results of clinical trials, RFM has been approved in the European Union for specific use in patients with Lennox-Gastaut syndrome and therefore represents a new therapeutic alternative with which to offset the medication resistance of this epileptic encephalopathy.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
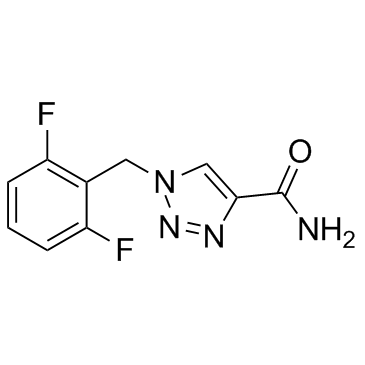 |
卢非酰胺
CAS:106308-44-5 |
C10H8F2N4O |
|
Dose-dependent pharmacokinetics and brain penetration of ruf...
2015-02-20 [Eur. J. Pharm. Sci. 68 , 106-13, (2015)] |
|
Toxicological screening of human plasma by on-line SPE-HPLC-...
2015-06-01 [Biomed. Chromatogr. 29 , 935-52, (2015)] |
|
Rufinamide for the treatment of Lennox-Gastaut syndrome.
2011-04-01 [Expert Opin. Pharmacother. 12(5) , 801-6, (2011)] |
|
Treatment of malignant migrating partial epilepsy of infancy...
2011-03-01 [Epileptic Disord. 13(1) , 18-21, (2011)] |
|
Bioavailability of three rufinamide oral suspensions compare...
2011-01-01 [Clin. Ther. 33(1) , 146-57, (2011)] |