Differential effects of the tyrosine kinase inhibitors on collagen type 1-induced platelet aggregation and adhesion to this protein.
U Kralisz, C S Cierniewski
文献索引:Thromb. Res. 86(4) , 287-99, (1997)
全文:HTML全文
摘要
Herbimycin A, lavendustin A, and methyl 2,5-dihydroxycinnamate were used to study the role of protein tyrosine kinases in collagen-platelet interaction. All three compounds produced a concentration dependent inhibition of platelet aggregation induced by collagen type I, characterized by values of IC50 equaled to 0.9, 10.0, and 5.0 microM, respectively. This effect was accompanied by strong inhibition of phosphorylation of p125FAK, p90, p72syk, p60c-arc, and p56lyn. In the absence of the inhibitors, phosphorylation of these proteins is evoked by aggregation of platelets. In addition to the antiaggregatory effect, the tyrosine kinase inhibitors reduced adhesion of platelets to collagen although to much lower extent than aggregation. Platelets which adhered to collagen showed also the presence of phosphorylated p125FAK, p90, p72syk, p60c-arc, and p56lyn. Of these proteins, the extent of phosphorylation of p90 was particularly high. Adhesion of platelets was associated with inhibition of phosphorylation of p125FAK, p60c-arc, and p56lyn only when high concentration of lavendustin A and methyl 2,5-dihydroxycinnamate were used. Herbimycin A did not affect adhesion-evoked protein tyrosine phosphorylation. Phosphorylation of p90 and p72syk was not affected by inhibitors. This study indicates that collagen type I can induce different transmembrane signalling dependent upon whether platelet aggregates formation or adhesion of platelets to this protein occurs.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
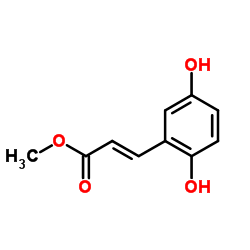 |
2,5-二羟基肉桂酸甲酯
CAS:63177-57-1 |
C10H10O4 |
|
Direct inhibition of the hexose transporter GLUT1 by tyrosin...
2001-01-23 [Biochemistry 40(3) , 777-90, (2001)] |
|
Mechanism of topoisomerase II inhibition by staurosporine an...
1996-10-18 [J. Biol. Chem. 271(42) , 26418-23, (1996)] |
|
Signal pathways that transduce growth factor-stimulated mito...
1998-07-01 [Bone 23(1) , 17-26, (1998)] |
|
Suppression of tyrosine kinase activity inhibits [3H]thymidi...
1997-07-01 [J. Clin. Endocrinol. Metab. 82(7) , 2143-7, (1997)] |
|
Regulation of the renal Na-HCO3 cotransporter: IX. Modulatio...
1998-10-16 [Regul. Pept. 77(1-3) , 155-61, (1998)] |