Probing the peptidylglycine alpha-hydroxylating monooxygenase active site with novel 4-phenyl-3-butenoic acid based inhibitors.
Emma Langella, Sébastien Pierre, Wadih Ghattas, Michel Giorgi, Marius Réglier, Michele Saviano, Luciana Esposito, Renaud Hardré
文献索引:ChemMedChem 5(9) , 1568-76, (2010)
全文:HTML全文
摘要
Specific inhibition of the copper-containing peptidylglycine alpha-hydroxylating monooxygenase (PHM), which catalyzes the post-translational modification of peptides involved in carcinogenesis and tumor progression, constitutes a new approach for combating cancer. We carried out a structure-activity study of new compounds derived from a well-known PHM substrate analogue, the olefinic compound 4-phenyl-3-butenoic acid (PBA). We designed, synthesized, and tested various PBA derivatives both in vitro and in silico. We show that it is possible to increase PBA affinity for PHM by appropriate functionalization of its aromatic nucleus. Compound 2 d, for example, bears a meta-benzyloxy substituent, and exhibits better inhibition features (K(i)=3.9 microM, k(inact)/K(i)=427 M(-1) s(-1)) than the parent PBA (K(i)=19 microM, k(inact)/K(i)=82 M(-1) s(-1)). Docking calculations also suggest two different binding modes for PBA derivatives; these results will aid in the development of further PHM inhibitors with improved features.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
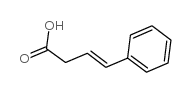 |
4-苯基-3-丁烯酸
CAS:1914-58-5 |
C10H10O2 |
|
Synthesis and antimalarial and antituberculosis activities o...
2012-02-15 [Bioorg. Med. Chem. 20 , 1482-93, (2012)] |
|
4-Phenyl-3-butenoic acid, an in vivo inhibitor of peptidylgl...
1990-04-30 [Eur. J. Biochem. 189(2) , 363-8, (1990)] |
|
Selective mechanism-based inactivation of peptidylglycine al...
1997-06-01 [Biochem. Pharmacol. 53(11) , 1695-702, (1997)] |
|
Fragrance material review on 4-phenyl-3-buten-2-ol.
2012-09-01 [Food Chem. Toxicol. 50 Suppl 2 , S120-3, (2012)] |
|
Reversal of the transformed phenotype and inhibition of pept...
2004-12-01 [Mol. Carcinog. 41(4) , 231-46, (2004)] |