Formation of 6-dimethylcarbamyloxy-dGuo, 6-dimethylamino-dGuo and 4-dimethylamino-dThd following in vitro reaction of dimethylcarbamyl chloride with calf thymus DNA and 6-diethylcarbamyloxy-dGuo following in vitro reaction of diethylcarbamyl chloride with calf thymus DNA.
A Segal, J J Solomon, U Maté, B L Van Duuren
文献索引:Chem. Biol. Interact. 40(2) , 209-31, (1982)
全文:HTML全文
摘要
The rodent carcinogens dimethylcarbamyl chloride (DMCC) and diethylcarbamyl chloride (DECC) react with dGuo (pH 7.0-7.5, 37 degrees C, 4 h) to form the O6-acyl derivatives 6-dimethylcarbamyloxy-2'-deoxyguanosine (6-DMC-dGuo) and 6-diethylcarbamyloxy-2'-deoxyguanosine (6-DEC-dGuo), respectively. Reaction of DMCC with dThd under identical conditions yielded 4-dimethylamino-thymidine (4-DMA-dThd). Compounds 6-DMC-dGuo and 6-DEC-dGuo undergo a nucleophilic aromatic substitution reaction with dimethylamine (DMA) to form 6-dimethylamino-2'-deoxyguanosine (6-DMA-dGuo) via displacement of the C-6 dialkylcarbamyloxy moiety. The substitution reaction did not take place when diethylamine or NH3 were substituted for DMA. The structures of the new compounds 6-DMC-dGuo, 6-DEC-dGuo, 4-DMA-dThd and 6-DMA-dGuo were deduced from chemical analyses and syntheses, UV and nuclear magnetic resonance (NMR) spectra and electron impact, isobutane chemical ionization and source insertion isobutane chemical ionization mass spectra. It was postulated that 4-DMA-dThd was formed following reaction of the transient intermediate 4-DMC-dThd with DMA formed by hydrolysis of DMCC. Calf thymus DNA was reacted in vitro with DMCC (pH 7.0-7.5, 37 degrees C, 4 h) and the modified DNA hydrolyzed enzymatically to 2'-deoxynucleosides. Compounds 6-DMC-dGuo, 4-DMA-dThd and 6-DMA-dGuo were identified in the hydrolysate by high-pressure liquid chromatography (HPLC). In an identical manner 6-DEC-dGuo was identified following in vitro reaction of DECC with calf thymus DNA. Compounds 6-DEC-dGuo and 6-DMC-dGuo possess novel structures with respect to the types of adducts known to be formed between carcinogens and bases in DNA. The implications of these findings with respect to chemical mutagenesis and carcinogenesis is discussed. The structural relationship between N4-dimethyl-5-methylcytosine (4-dimethylamino-Thy) formed in DNA following in vitro reaction with DMCC and 5-methylcytosine, the only modified base found in vertebrate DNA is noted.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
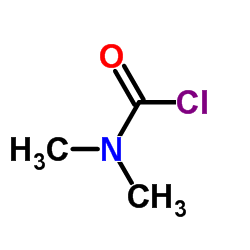 |
二甲氨基甲酰氯
CAS:79-44-7 |
C3H6ClNO |
|
Phenyldichlorophosphate as an aid in studies of decarbamylat...
1988-05-01 [Anal. Biochem. 170(2) , 451-5, (1988)] |
|
Dimethylcarbamoyl chloride.
2011-01-01 [Rep. Carcinog. 12 , 171-2, (2011)] |
|
Dimethylcarbamoyl chloride.
2004-01-01 [Rep. Carcinog. 11 , III107, (2004)] |
|
Dimethylcarbamoyl chloride.
2002-01-01 [Rep. Carcinog. 10 , 106-7, (2002)] |
|
Dimethylcarbamoyl chloride.
1999-01-01 [IARC Monogr. Eval. Carcinog. Risks Hum. 71 Pt 2 , 531-43, (1999)] |