Theoretical insight into stereoselective reaction mechanisms of 2,4-pentanediol-tethered ketene-olefin [2 + 2] cycloaddition.
Katsumasa Kamiya, Toru Matsui, Takashi Sugimura, Yasuteru Shigeta
文献索引:J. Phys. Chem. A 116(4) , 1168-75, (2012)
全文:HTML全文
摘要
We report ab initio molecular dynamics calculations based on density functional theory performed on an intramolecular [2 + 2] cycloaddition between ketene and olefin linked with a 2,4-pentanediol (PD) tether. We find that the encounter of the ketene and olefin moieties could be prearranged in the thermal equilibrated state before the cycloaddition. The reaction mechanism is found to be stepwise, similar to that of intermolecular ketene [2 + 2] cycloadditions with ordinary alkenes. A distinct feature of the reaction pathway for a major diastereoisomer is a differential activation free energy of about 1.5 kcal/mol, including 2.8 kcal/mol as the differential activation entropy, with a transition state consisting of a flexible nine-membered ring in the olefin-PD-ketene moiety. This theoretical study provides a reasonable explanation for the strict stereocontrollability of the PD-tethered ketene-olefin cycloaddition, irrespective of reaction types or conditions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
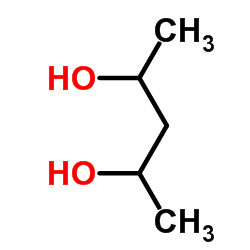 |
2,4-戊二醇
CAS:625-69-4 |
C5H12O2 |
|
Brønsted acid catalyzed asymmetric reduction of ketones and ...
2010-05-21 [Org. Lett. 12(10) , 2294-7, (2010)] |
|
Highly efficient synthesis of enantiopure diacetylated C(2)-...
2006-08-07 [Chemistry 12 , 6053, (2006)] |
|
2,4-Pentanediolate as an alkoxide/diketonate "hybrid" ligand...
2011-12-05 [Inorg. Chem. 50(23) , 12126-32, (2011)] |
|
Lack of selectivity between anesthetic stereoisomers for an ...
1990-07-09 [Biochim. Biophys. Acta 1026(1) , 40-2, (1990)] |