Kinetic studies on the reaction of chlorosulfonyl isocyanate with monofluoroalkenes: experimental evidence for both stepwise and concerted mechanisms and a pre-equilibrium complex on the reaction pathway.
Dale F Shellhamer, Summer A Bunting, Kelli R Hickle, Parker C Horn, Jacob C Milligan, Danielle E Shipowick, Lincoln B Smith, David J Vandenbroek, Marc C Perry, Jerry A Boatz
文献索引:J. Org. Chem. 78(2) , 246-52, (2013)
全文:HTML全文
摘要
Chlorosulfonyl isocyanate (CSI) is reported to react with hydrocarbon alkenes by a stepwise dipolar pathway to give N-chlorosulfonyl-β-lactams that are readily reduced to β-lactams. Substitution of a vinyl hydrogen for a vinyl fluorine changes the dynamics for reaction with CSI so that a concerted pathway is favored. Rate constants were measured for reactions of CSI with monofluoroalkenes and some hydrocarbon alkenes. Activation parameters for two hydrocarbon alkenes and two monofluoroalkenes support this change in mechanism. A plot generated from the natural log of rate constants vs ionization potentials (IP) indicates that fluoroalkenes with IP values >8.9 eV react by a concerted process. Electron-rich monofluoroalkenes with IP values <8.5 eV were found to react by a single-electron transfer (SET) pathway. Hydrocarbon alkenes were also found to react by this dipolar stepwise SET intermediate rather than the previously accepted stepwise dipolar pathway. Data support a pre-equilibrium complex on the reaction pathway just before the rate-determining step of the concerted pathway and a SET intermediate for the stepwise reactions. When the reactions are carried out at lower temperatures, the equilibrium shifts toward the complex or SET intermediate enhancing the synthetic utility of these reactions. Kinetic data also support formation of a planar transition state rather than the orthogonal geometry as reported for ketene [2 + 2] cycloadditions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
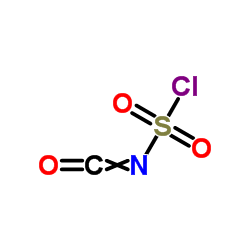 |
氯磺酰异氰酸酯
CAS:1189-71-5 |
CClNO3S |
|
Synthesis and carbonic anhydrase inhibitory properties of su...
2013-06-01 [Bioorg. Med. Chem. 21(11) , 2925-31, (2013)] |
|
Synthesis and characterization of a new class of inhibitors ...
1993-06-15 [J. Biol. Chem. 268(17) , 12933-8, (1993)] |
|
Synthesis of (2R,5S)-dihydroxymethyl-(3R,4R)-dihydroxypyrrol...
2007-08-13 [Carbohydr. Res. 342(11) , 1502-9, (2007)] |
|
Inhibitors of acyl-CoA:cholesterol O-acyltransferase. 17. St...
1996-03-15 [J. Med. Chem. 39(6) , 1243-52, (1996)] |
|
Preparation of an asymmetric semipermeable membrane with ant...
1984-05-01 [Biomaterials 5(3) , 153-6, (1984)] |