Inhibition studies on LDH isoenzyme purified from Uromastix testes.
M U Javed, M A Waqar
文献索引:J. Enzym. Inhib. 10(3) , 187-93, (1996)
全文:HTML全文
摘要
An LDH isoenzyme was purified to homogeneity from uromastix testes and its inhibition spectrum towards known LDH isoenzyme inhibitors studied. Platinum compounds inhibited the enzyme in the forward reaction (pyruvate-->lactate) only, n-hexanediol and colchicine showed no inhibition and gossypol acetic acid (GAA) strongly inhibited both the forward and reverse reactions and the reactions were time-dependent. Oxalate caused non-competitive inhibition (Ki app = IC50 = 0.15 mM) of the forward reaction, NADH was more effective in blocking inhibition by GAA than pyruvate. This enzyme was also unable to use ketocaproic acid as a substrate.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
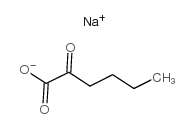 |
2-酮己酸 钠盐
CAS:13022-85-0 |
C6H9NaO3 |
|
Calcium uptake by bovine epididymal spermatozoa is regulated...
1989-04-01 [Biol. Reprod. 40(4) , 744-51, (1989)] |
|
Lactate dehydrogenase-C4 is involved in heparin- and NADH-de...
2002-04-01 [Andrologie 34(2) , 91-7, (2002)] |
|
GC-MS profiling of urinary organic acids evaluated as a quan...
1996-10-01 [Clin. Chem. 42(10) , 1609-15, (1996)] |
|
The stimulus-secretion coupling of amino acid-induced insuli...
1981-09-18 [Biochim. Biophys. Acta 677(1) , 32-8, (1981)] |
|
[Inhibition of postoperative muscular proteolysis by sodium ...
1985-01-01 [Ann. Fr. Anesth. Reanim. 4(4) , 351-4, (1985)] |