Stereospecificity of horseradish peroxidase.
M Angeles Gilabert, Lorena G Fenoll, Francisco García-Molina, Pedro A García-Ruiz, José Tudela, Francisco García-Cánovas, José N Rodríguez-López
文献索引:Biol. Chem. 385(12) , 1177-84, (2004)
全文:HTML全文
摘要
We report here on the stereospecificity observed in the action of horseradish peroxidase (HRPC) on monophenol and diphenol substrates. Several enantiomers of monophenols and o-diphenols were assayed: L-tyrosinol, D-tyrosinol, L-tyrosine, DL-tyrosine, D-tyrosine, L-dopa, DL-dopa, D-dopa, L-alpha-methyldopa, DL-alpha-methyldopa, DL-adrenaline, D-adrenaline, L-isoproterenol, DL-isoproterenol and D-isoproterenol. The electronic density at the carbon atoms in the C-1 and C-2 positions of the benzene ring were determined by NMR assays (delta1 and delta2). This value is related to the nucleophilic power of the oxygen atom of the hydroxyl groups and to its oxidation-reduction capacity. The spatial orientation of the ring substituents resulted in lower Km values for L- than for D-isomers. The kcat values for substrates capable of saturating the enzyme were lower for D- than for L-isomers, although both have the same delta1 and delta2 NMR values for carbons C-1 and C-2, and therefore the same oxidation-reduction potential. In the case of substrates that cannot saturate the enzyme, the values of the binding constant for compound II (an intermediate in the catalytic cycle) followed the order: L-isomer>DL-isomer>D-isomer. Therefore, horseradish peroxidase showed stereospecificity in its affinity toward its substrates (K m) and in their transformation reaction rates (k cat).
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
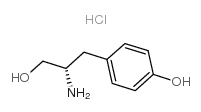 |
d-酪氨醇盐酸盐
CAS:40829-04-7 |
C9H14ClNO2 |
|
The double-length tyrosyl-tRNA synthetase from the eukaryote...
2011-06-03 [J. Mol. Biol. 409(2) , 159-76, (2011)] |
|
Selective killing of transformed cells by methotrexate with ...
1983-10-01 [Cancer Res. 43(10) , 4703-8, (1983)] |
|
Interaction of crystalline tyrosyl-tRNA synthetase with aden...
1984-03-15 [J. Mol. Biol. 173(4) , 477-85, (1984)] |