The role of halogen substitution in classical cannabinoids: a CB1 pharmacophore model.
Spyros P Nikas, Jolanta Grzybowska, Demetris P Papahatjis, Avgui Charalambous, Ali R Banijamali, Ravi Chari, Pusheng Fan, Therapia Kourouli, Sonyuan Lin, Albert J Nitowski, Gilbert Marciniak, Yan Guo, Xiuyan Li, Chia-Lin J Wang, Alexandros Makriyannis
文献索引:AAPS J. 6(4) , e30, (2004)
全文:HTML全文
摘要
The presence of halogens within the classical cannabinoid structure leads to large variations in the compounds' potencies and affinities for the CB1 receptors. To explore the structure activity relationships within this class of analogs we have used a series of halogen-substituted (-)-Delta8-tetrahydrocannabinol analogs and compared their affinities for the CB1 cannabinoid receptor. Our results indicate that halogen substitution at the end-carbon of the side chain leads to an enhancement in affinity with the bulkier halogens (Br, I) producing the largest effects. Conversely, 2-iodo substitution on the phenolic ring leads to a 2-fold reduction in affinity while iodo-substitution in the C1'-position of the side chain lowers the compound's affinity for CB1 by more than 8-fold. The pharmacophoric requirements resulting from halogen-substitution are explored using computer modeling methods.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
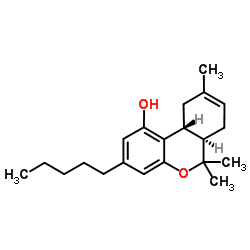 |
DEA限制
CAS:5957-75-5 |
C21H30O2 |
|
Effects of cannabinoids in membrane bilayers containing chol...
1999-08-20 [Biochim. Biophys. Acta 1420(1-2) , 252-65, (1999)] |
|
Receptor mechanism and antiemetic activity of structurally-d...
2007-06-01 [Eur. J. Pharmacol. 563(1-3) , 187-96, (2007)] |
|
Loss of THCCOOH from urine specimens stored in polypropylene...
2000-10-01 [J. Anal. Toxicol. 24(7) , 567-71, (2000)] |
|
Characterisation of the rat cerebella CB1 receptor using SR1...
1996-12-13 [Neurosci. Lett. 220(2) , 101-4, (1996)] |
|
Cannabinoids inhibit cellular respiration of human oral canc...
2010-01-01 [Pharmacology 85(6) , 328-35, (2010)] |