Chemical & Pharmaceutical Bulletin
2000-02-01
Fluorinative Beckmann fragmentation: fluorinative alpha-cleavage of cyclic ketoximes by diethylaminosulfur trifluoride.
M Kirihara, K Niimi, M Okumura, T Momose
文献索引:Chem. Pharm. Bull. 48(2) , 220-2, (2000)
全文:HTML全文
摘要
Diethylaminosulfur trifluoride reacted with cyclic ketoximes bearing substituent(s) that can stabilize a carbocation to cause fluorinative fragmentation, affording fluorinated carbonitrile. Ketoximes lacking such substituents afforded complex mixtures. However, the introduction of a sulfur functionality, which can stabilize a carbocation and can be easily removed from the reaction products, into the ketoxime was effective for producing the fluorinative fragmentation.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
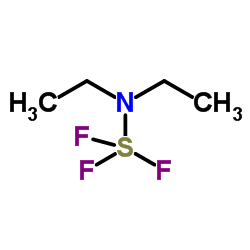 |
二乙氨基三氟化硫
CAS:38078-09-0 |
C4H10F3NS |
相关文献:
更多...
|
Rearrangement of homoallylic alcohols induced by DAST.
2006-05-11 [Org. Lett. 8 , 2091, (2006)] |
|
Synthesis of functionalized oxazolines and oxazoles with DAS...
2000-04-20 [Org. Lett. 2(8) , 1165-8, (2000)] |
|
Ring expansion of cyclic β-amino alcohols induced by diethyl...
2012-07-20 [J. Org. Chem. 77(14) , 6087-99, (2012)] |
|
Solid-phase chemical synthesis and in vitro biological evalu...
2012-11-01 [Steroids 77(13) , 1403-18, (2012)] |
|
Stereo- and regio-selectivity of diethylaminosulfur trifluor...
1983-09-16 [Carbohydr. Res. 121 , 51-60, (1983)] |