Probing a functional role of Glu87 and Trp89 in the lid of Humicola lanuginosa lipase through transesterification reactions in organic solvent.
M Holmquist, IG Clausen, S Patkar, A Svendsen, K Hult
文献索引:J. Protein Chem. 14(4) , 217-24, (1995)
全文:HTML全文
摘要
To reveal the functional role of Glu87 and Trp89 in the lid of Humicola lanuginosa lipase, site-directed mutagenesis at Glu87 and Trp89 was carried out. The catalytic performance of wild-type and mutated lipases was studied in transesterification reactions in cyclohexane at a controlled water activity. Two different acyl donors were used in the investigation: tributyrin, a natural substrate for a lipase, and vinyl butyrate, an activated ester suitable for fast and efficient lipase-catalyzed transformations in preparative organic synthesis. As acyl acceptor 1-heptanol was used. The Glu87Ala mutation decreased the Vmax,app value with tributyrin and vinyl butyrate by a factor of 1.5 and 2, respectively. The Km,app for tributyrin was not affected by the Glu87Ala mutation, but the Km,app for vinyl butyrate increased twofold compared to the wild-type lipase. Changing Trp89 into a Phe residue afforded an enzyme with a 2.7- and 2-fold decreased Vmax,app with the substrates tributyrin and vinyl butyrate, respectively, compared to the wild-type lipase. No significant effects on the Km,app values for tributyrin or vinyl butyrate were seen as a result of the Trp89Phe mutation. However, the introduction of a Glu residue at position 89 in the lid increased the Km,app for tributyrin and vinyl butyrate by a factor of > 5 and 2, respectively. The Trp89Glu mutated lipase could not be saturated with tributyrin within the experimental conditions (0-680 mM) studied here. With vinyl butyrate as a substrate the Vmax,app was only 6% of that obtained with wild-type enzyme.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
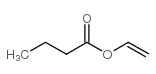 |
正丁酸乙烯酯
CAS:123-20-6 |
C6H10O2 |
|
Solid-state fermentation as a potential technique for estera...
2015-11-01 [Extremophiles 19 , 1121-32, (2015)] |
|
Enhanced productivity of electrospun polyvinyl alcohol nanof...
2012-08-01 [J. Biosci. Bioeng. 114(2) , 204-8, (2012)] |
|
Lipase-catalysed hydrolysis of short-chain substrates in sol...
2001-11-30 [Biochim. Biophys. Acta 1534(1) , 34-44, (2001)] |
|
Distinction between esterases and lipases: a kinetic study w...
2002-07-01 [Lipids 37(7) , 653-62, (2002)] |
|
Substrate specificity and kinetic properties of enzymes belo...
2005-12-30 [Biochim. Biophys. Acta 1738(1-3) , 29-36, (2005)] |