Two-step hard acid deprotection/cleavage procedure for solid phase peptide synthesis.
M Nomizu, Y Inagaki, T Yamashita, A Ohkubo, A Otaka, N Fujii, P P Roller, H Yajima
文献索引:Int. J. Pept. Protein Res. 37(2) , 145-52, (1991)
全文:HTML全文
摘要
A new two-step deprotection/cleavage procedure for t-butoxycarbonyl (Boc) based solid phase peptide synthesis is reported. First the protective groups are removed from 4-(oxymethyl)-phenylacetamidomethyl (PAM) resin attached peptide with the weak hard acid, trimethylsilyl bromide-thioanisole/trifluoroacetic acid (TFA). In the second step, the peptide is cleaved from the resin with a stronger hard acid such as trimethylsilyl trifluoromethanesulfonate in TFA or with HF. The method is also shown to deformylate Nin-formyltryptophan moiety efficiently. The usefulness of this procedure for practical solid phase peptide synthesis is demonstrated by comparison with other deprotection methods in the synthesis of urotensin II and human endothelin.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
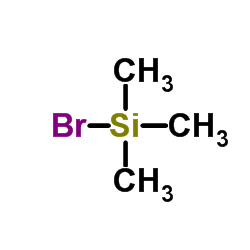 |
三甲基溴硅烷
CAS:2857-97-8 |
C3H9BrSi |
|
EPR and NMR spectroscopies provide input on the coordination...
2015-06-01 [J. Biol. Inorg. Chem. 20 , 719-27, (2015)] |
|
Position and length of fatty acids strongly affect receptor ...
2014-11-01 [ChemMedChem 9(11) , 2463-74, (2014)] |
|
Reduced vascular endothelial growth factor and capillary den...
2014-07-01 [Brain Pathol. 24(4) , 334-43, (2014)] |
|
The chemoselective and efficient deprotection of silyl ether...
2008-06-21 [Org. Biomol. Chem. 6(12) , 2168-72, (2008)] |
|
Capillary gas chromatography of hexadecylphosphocholine in C...
1995-05-01 [Anal. Biochem. 227(1) , 246-50, (1995)] |