CD38 cleavage in fMLP- and IL-8-induced chemotaxis is dependent on p38 MAP kinase but independent of p44/42 MAP kinase.
Tsuyoshi Fujita, Khalid H Zawawi, Hidemi Kurihara, Thomas E Van Dyke
文献索引:Cell. Signal. 17(2) , 167-75, (2005)
全文:HTML全文
摘要
In this study, we examined the mechanism by which CD38 cleavage is regulated through the mitogen-activated protein (MAP) kinases after stimulation by fMLP and interleukin-8 (IL-8) in neutrophils. Both fMLP and IL-8 increased chemotaxis and decreased CD38 protein in neutrophils, but did not change CD38 mRNA levels. Both fMLP and IL-8 increased CD38 in supernatants, which was inhibitable with PMSF. fMLP stimulation resulted in phosphorylation of p38 MAP kinase and p42/44 MAP kinase (ERK). SB20358, a p38 MAP kinase inhibitor, down-regulated neutrophil chemotaxis. Conversely, PD98059, an ERK inhibitor, did not influence chemotaxis to either agonist. The addition of SB20358 blocked the decrease of CD38 on neutrophils and the increase in supernatants induced by fMLP or IL-8, whereas PD98059 did not. These findings suggest that CD38-mediated chemotaxis to fMLP or IL-8 is characterized by proteolytic cleavage of CD38 and signaling through p38 MAP kinase. Activation of the protease for cleavage appears to be a postreceptor event that is dependent on p38 MAP kinase signaling.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
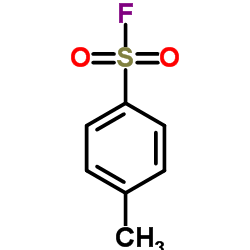 |
对甲苯磺酰气氟
CAS:455-16-3 |
C7H7FO2S |
|
Microplate-based method to screen inhibitors of isozymes of ...
2014-12-01 [J. Enzyme Inhib. Med. Chem. 29(6) , 836-9, (2014)] |
|
Assessment of the enzymatic activity and inhibition using HP...
2010-02-01 [J. Chromatogr. Sci. 48(2) , 150-5, (2010)] |
|
Determination of the cytotoxic effect of Clostridium histoly...
2005-08-01 [FEMS Immunol. Med. Microbiol. 45(2) , 137-42, (2005)] |
|
Longistatin is an unconventional serine protease and induces...
2012-01-01 [Mol. Biochem. Parasitol. 182(1-2) , 45-53, (2012)] |
|
Esterase inhibition in tadpoles of Scinax fuscovarius (Anura...
2010-09-01 [Environ. Sci. Pollut. Res. Int. 17(8) , 1411-21, (2010)] |