Route-specific differences in distribution characteristics of octamethylcyclotetrasiloxane in rats: analysis using PBPK models.
Ramesh Sarangapani, Justin Teeguarden, Melvin E Andersen, Richard H Reitz, Kathleen P Plotzke
文献索引:Toxicol. Sci. 71(1) , 41-52, (2003)
全文:HTML全文
摘要
Octamethylcyclotetrasiloxane (D(4)) is used in selected consumer products and has a potential for human exposure from multiple routes. Here we develop a physiologically based pharmacokinetic (PBPK) model to describe the tissue dosimetry, plasma concentration, and clearance in the rat following inhalation and dermal, oral, and iv exposure. An initial multiroute PBPK model, based on a previously published inhalation PBPK model for D(4), provided excellent fits to the observed concentration time course of D(4) metabolites in urine and D(4) exhalation rate following dermal exposures. However, the pharmacokinetics of D(4), following oral and iv exposure, were sensitive to the mode of entry into the blood compartment. A refined model, describing delivery of D(4) from the GI tract to the nonexchangeable/deep blood compartment, provided the best fits to observed plasma D(4), exhaled D(4), and D(4) metabolites excreted in the urine following oral exposure. Pharmacokinetics following iv administration was best described by delivery of D(4) directly into the deep blood compartment, possibly reflecting a kinetically identifiable characteristic of the administration of D(4) as an emulsion for the intravenous route of exposure. This model-based analysis indicates that the pharmacokinetics of D(4) delivered by the inhalation or dermal routes is similar, and is different from the iv or oral delivery routes.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
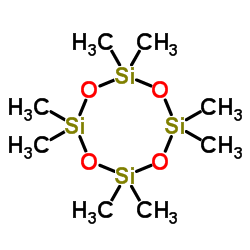 |
八甲基环四硅氧烷
CAS:556-67-2 |
C8H24O4Si4 |
|
Siloxane treatment by adsorption into porous materials.
2009-09-01 [Environ. Technol. 30(10) , 1073-83, (2009)] |
|
Allometric relationships to liver tissue concentrations of c...
2014-07-01 [Environ. Pollut. 190 , 109-14, (2014)] |
|
[Analysis of the volatile oils chemical constituents of root...
2008-05-01 [Zhong Yao Cai 31(5) , 677-8, (2008)] |
|
A two-generation reproductive toxicity study of octamethylcy...
2007-02-01 [Reprod. Toxicol. 23(2) , 202-15, (2007)] |
|
In vitro and in vivo percutaneous absorption of 14C-octameth...
2008-03-01 [Regul Toxicol Pharmacol 50(2) , 239-48, (2008)] |