Purification and characterization of fumarase from Corynebacterium glutamicum.
Tomoko Genda, Shoji Watabe, Hachiro Ozaki
文献索引:Biosci. Biotechnol. Biochem. 70(5) , 1102-9, (2006)
全文:HTML全文
摘要
Fumarase (EC 4.2.1.2) from Corynebacterium glutamicum (Brevibacterium flavum) ATCC 14067 was purified to homogeneity. Its amino-terminal sequence (residues 1 to 30) corresponded to the sequence (residues 6 to 35) of the deduced product of the fumarase gene of C. glutamicum (GenBank accession no. BAB98403). The molecular mass of the native enzyme was 200 kDa. The protein was a homotetramer, with a 50-kDa subunit molecular mass. The homotetrameric and stable properties indicated that the enzyme belongs to a family of Class II fumarase. Equilibrium constants (K(eq)) for the enzyme reaction were determined at pH 6.0, 7.0, and 8.0, resulting in K(eq)=6.4, 6.1, and 4.6 respectively in phosphate buffer and in 16, 19, and 17 in non-phosphate buffers. Among the amino acids and nucleotides tested, ATP inhibited the enzyme competitively, or in mixed-type, depending on the buffer. Substrate analogs, meso-tartrate, D-tartrate, and pyromellitate, inhibited the enzyme competitively, and D-malate in mixed-type.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
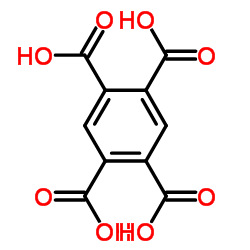 |
均苯四甲酸
CAS:89-05-4 |
C10H6O8 |
|
Short-column anion-exchange chromatography for soil and peat...
2015-08-21 [J. Chromatogr. A. 1408 , 72-7, (2015)] |
|
Benzenepolycarboxylic acids with potential anti-hemorrhagic ...
2011-12-01 [Bioorg. Med. Chem. 19 , 7000-2, (2011)] |
|
Effects of natural organic matter model compounds on the tra...
2010-04-01 [Water Res. 44(7) , 2125-32, (2010)] |
|
New support for the affinity chromatography of hemoglobin.
1997-06-20 [J. Chromatogr. B. Biomed. Sci. Appl. 694(1) , 39-48, (1997)] |
|
Pyrones to pyrans: enantioselective radical additions to acy...
2006-10-18 [J. Am. Chem. Soc. 128(41) , 13346-7, (2006)] |