Bioorganic & Medicinal Chemistry
2008-07-15
Synthesis and proteasome inhibition of glycyrrhetinic acid derivatives.
Li Huang, Donglei Yu, Phong Ho, Keduo Qian, Kuo-Hsiung Lee, Chin-Ho Chen, Li Huang, Donglei Yu, Phong Ho, Keduo Qian, Kuo-Hsiung Lee, Chin-Ho Chen
文献索引:Bioorg. Med. Chem. 16 , 6696-6701, (2008)
全文:HTML全文
摘要
This study discovered that glycyrrhetinic acid inhibited the human 20S proteasome at 22.3microM. Esterification of the C-3 hydroxyl group on glycyrrhetinic acid with various carboxylic acid reagents yielded a series of analogs with marked improved potency. Among the derivatives, glycyrrhetinic acid 3-O-isophthalate (17) was the most potent compound with IC(50) of 0.22microM, which was approximately 100-fold more potent than glycyrrhetinic acid.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
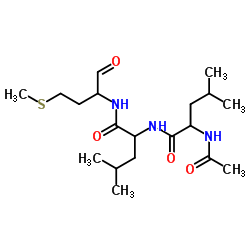 |
钙蛋白酶抑制剂II(进口)
CAS:136632-32-1 |
C19H35N3O4S |
相关文献:
更多...
|
Compartmentalization of the chick cerebellar cortex based on...
2015-09-01 [J. Comp. Neurol. 523 , 1886-912, (2015)] |
|
Targeting microparticle biogenesis: a novel approach to the ...
2015-01-01 [Curr. Cancer Drug Targets 15 , 205-14, (2015)] |
|
Increased μ-Calpain Activity in Blasts of Common B-Precursor...
2015-01-01 [PLoS ONE 10 , e0136615, (2015)] |
|
Novel targets for Huntington's disease in an mTOR-independen...
2008-05-01 [Nat. Chem. Biol. 4 , 295-305, (2008)] |
|
The cyclin-dependent kinase activator, Spy1A, is targeted fo...
2009-01-30 [J. Bacteriol. 284 , 2617-27, (2009)] |