Efficient large-scale synthesis of BILN 2061, a potent HCV protease inhibitor, by a convergent approach based on ring-closing metathesis.
Nathan K Yee, Vittorio Farina, Ioannis N Houpis, Nizar Haddad, Rogelio P Frutos, Fabrice Gallou, Xiao-Jun Wang, Xudong Wei, Robert D Simpson, Xuwu Feng, Victor Fuchs, Yibo Xu, Jonathan Tan, Li Zhang, Jinghua Xu, Lana L Smith-Keenan, Jana Vitous, Michael D Ridges, Earl M Spinelli, Michael Johnson, Kai Donsbach, Thomas Nicola, Michael Brenner, Eric Winter, Paul Kreye, Wendelin Samstag
文献索引:J. Org. Chem. 71 , 7133, (2006)
全文:HTML全文
摘要
A multistep scalable synthesis of the clinically important hepatitis C virus (HCV) protease inhibitor BILN 2061 (1) is described. The synthesis is highly convergent and consists of two amide bond formations, one etherification, and one ring-closing metathesis (RCM) step, using readily available building blocks 2-5. The optimization of each step is described at length. The main focus of the paper is the study of the RCM step and the description of the main problems faced when scaling up to pilot scale this highly powerful but very challenging synthetic operation. Eventually, the RCM reaction was smoothly scaled up to produce >400 kg of cyclized product.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
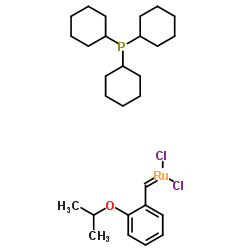 |
二氯(邻异丙氧基苯基亚甲基)(三环己基膦)钌
CAS:203714-71-0 |
C28H45Cl2OPRu |
|
Ruthenium-based heterocyclic carbene-coordinated olefin meta...
2010-03-10 [Chem. Rev. 110 , 1746, (2010)] |
|
Olefin Metathesis and Beyond A list of abbreviations can be ...
2000-10-01 [Angew. Chem. Int. Ed. Engl. 39 , 3012, (2000)] |
|
The development of L2X2Ru=CHR olefin metathesis catalysts: a...
2001-01-01 [Acc. Chem. Res. 34 , 18, (2001)] |
|
Sequential catalysis: a metathesis/dihydroxylation sequence.
2006-03-13 [Angew. Chem. Int. Ed. Engl. 45 , 1900, (2006)] |
|
SYNTHESIS AND SPECTROSCOPIC DIFFERENTIATION OF 2- AND 4-ALKO...
2014-08-15 [Heterocycles 63 , 5-8, (2004)] |