Regioselective deprotection of orthobenzoates for the synthesis of inositol phosphates.
Joanna M Swarbrick, Samuel Cooper, Geert Bultynck, Piers R J Gaffney
文献索引:Org. Biomol. Chem. 7(8) , 1709-15, (2009)
全文:HTML全文
摘要
Synthetic myo-inositol 1,4,5-triphosphate, Ins(1,4,5)P(3), and myo-inositol 1,3,4,5-tetraphosphate, Ins(1,3,4,5)P(4), continue to be valuable in biological studies. Inositol orthoesters have proved an important class of intermediate to access these compounds. We investigated the ability of steric bulk from a 4-O protecting group to direct DIBAL-H reduction of inositol orthobenzoates to generate the natural Ins(1,4,5)P(3) precursor 2,3,6-O-tribenzyl myo-inositol. Introduction of an equatorial 4-C-methyl group imparts totally selective reduction and we report the synthesis of novel 4-C-methyl-Ins(1,4,5)P(3) and 4-C-methyl-Ins(1,3,4,5)P(4).
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
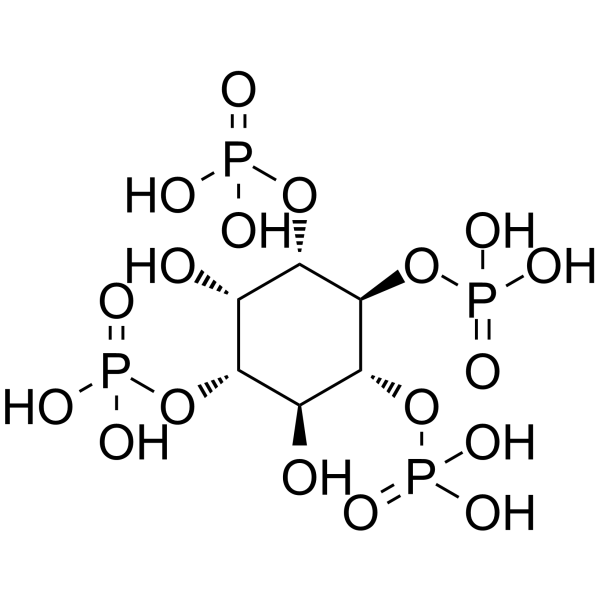 |
D-肌醇-1,3,4,5-四(磷酸)铵盐
CAS:102850-29-3 |
C6H16O18P4 |
|
IP3 3-kinase B controls hematopoietic stem cell homeostasis ...
2015-04-30 [Blood 125 , 2786-97, (2015)] |
|
Cell signaling. The art of the soluble.
2007-05-11 [Science 316(5826) , 845-6, (2007)] |
|
Interaction of the brain-specific protein p42IP4/centaurin-a...
2006-07-01 [J. Neurochem. 98(2) , 343-54, (2006)] |
|
Inositol 1,4,5-trisphosphate 3-kinase B is a negative regula...
2009-04-15 [J. Immunol. 182(8) , 4696-704, (2009)] |
|
Beyond IP3: roles for higher order inositol phosphates in im...
2008-02-15 [Cell Cycle 7(4) , 463-7, (2008)] |