Synthesis and anticancer activities of fatty acid analogs of podophyllotoxin.
Jamal Mustafa, Shabana I Khan, Guoyi Ma, Larry A Walker, Ikhlas A Khan
文献索引:Lipids 39(2) , 167-72, (2004)
全文:HTML全文
摘要
Derivatives of podophyllotoxin were prepared by coupling 10 FA with the C4-alpha-hydroxy function of podophyllotoxin. The coupling reactions between FA and podophyllotoxin were carried out by dicyclohexylcarbodiimide in the presence of a catalytic amount of dimethylaminopyridine to produce quantitative yields of desired products. FA incorporated were the following: 10-hydroxydecanoic, 12-hydroxydodecanoic, 15-hydroxypentadecanoic, 16-hydroxyhexadecanoic, 12-hydroxyoctadec-Z-9-enoic, eicosa-Z-5,8,11,14-tetraenoic, eicosa-Z-8,11, 14-trienoic, eicosa-Z-11,14-dienoic, eicosa-Z-11-enoic, and eicosanoic acids. Spectroscopic studies confirmed the formation of the desired products. New molecules were investigated for their in vitro anticancer activity against a panel of human cancer cell lines including SK-MEL, KB, BT-549, SK-OV-3 (solid tumors), and HL-60 (human leukemia) cells. Most of the analogs were cytotoxic against cancerous cells, whereas no effect was observed against normal cells, unlike the parent compound podophyllotoxin, the use of which is limited due to its severe side effects.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
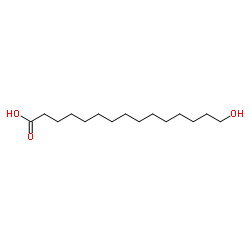 |
15-羟基十酸
CAS:4617-33-8 |
C15H30O3 |
|
Profiling of secondary metabolites in root exudates of Arabi...
2014-12-01 [Phytochemistry 108 , 35-46, (2014)] |
|
Matrix-assisted laser desorption/ionization mass spectrometr...
2014-12-01 [Plant J. 80(5) , 926-35, (2014)] |
|
Identification of the peroxisomal beta-oxidation enzymes inv...
2004-06-01 [J. Lipid Res. 45(6) , 1104-11, (2004)] |
|
Identification and functional characterization of a new CYP2...
2001-08-01 [Mol. Pharmacol. 60(2) , 382-7, (2001)] |
|
Preparation of 15-hydroxypentadecanoic acid by means of cond...
[Can. J. Chem. 46(23) , 3767-3770, (1968)] |