Conformational change of the E-F interhelical loop in the M photointermediate of bacteriorhodopsin.
Leonid S Brown, Richard Needleman, Janos K Lanyi
文献索引:J. Mol. Biol. 317(3) , 471-8, (2002)
全文:HTML全文
摘要
The conformation of the structured EF interhelical loop of bacteriorhodopsin and its change in the M photointermediate were assessed by measuring the rate of reaction of 16 single engineered cysteine residues along the loop with water-soluble sulfhydryl reagents. The exposure to the bulk in the unilluminated state determined with the cysteine reaction correlated well with the degree of access to water calculated from the crystallographic structure of the loop. The EF-loop should be affected by the well-known outward tilt of helix F in the M and N intermediates of the photocycle. A second mutation in each cysteine mutant, the D96N residue replacement, allowed full conversion to the M state by illumination. The reaction rates measured under these conditions indicated that buried residues tend to become more exposed, and exposed residues become more buried in M. This is to be expected from tilt of helix F. However, the observation of increased exposure of four residues near the middle of the loop, where steric effects are only from other loop residues, indicate that the conformation of the EF-loop itself is changed. Thus, the motion of the loop in M is more complex than expected from simple tilt of helix F, and may include rotation that unwinds its twist.Copyright 2002 Elsevier Science Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
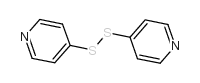 |
4,4'-二吡啶基二硫
CAS:2645-22-9 |
C10H8N2S2 |
|
Oxidative stress and lipid peroxidation are upstream of amyl...
2015-12-01 [Neurobiol. Dis. 84 , 109-19, (2015)] |
|
Molecularly imprinted tunable binding sites based on conjuga...
2009-07-01 [J. Am. Chem. Soc. 131(25) , 8833-8, (2009)] |
|
Quick measurement of protein sulfhydryls with Ellman's reage...
2002-07-01 [Anal. Bioanal. Chem 373(4-5) , 266-76, (2002)] |
|
Sulfhydryl modification by 4,4'-dithiodipyridine induces cal...
2003-11-01 [Arch. Toxicol. 77(11) , 630-7, (2003)] |
|
O6-alkylguanine-DNA alkyltransferase: low pKa and high react...
2003-09-23 [Biochemistry 42(37) , 10965-70, (2003)] |