Pyrene-modified PNAs: Stacking interactions and selective excimer emission in PNA2DNA triplexes.
Alex Manicardi, Lucia Guidi, Alice Ghidini, Roberto Corradini
文献索引:Beilstein J. Org. Chem. 10(1) , 1495-1503, (2014)
全文:HTML全文
摘要
Pyrene derivatives can be incorporated into nucleic acid analogs in order to obtain switchable probes or supramolecular architectures. In this paper, peptide nucleic acids (PNAs) containing 1 to 3 1-pyreneacetic acid units (PNA1-6) with a sequence with prevalence of pyrimidine bases, complementary to cystic fibrosis W1282X point mutation were synthesized. These compounds showed sequence-selective switch-on of pyrene excimer emission in the presence of target DNA, due to PNA2DNA triplex formation, with stability depending on the number and positioning of the pyrene units along the chain. An increase in triplex stability and a very high mismatch-selectivity, derived from combined stacking and base-pairing interactions, were found for PNA2, bearing two distant pyrene units.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
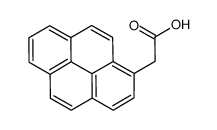 |
1-芘乙酸
CAS:64709-55-3 |
C18H12O2 |
|
Autonomous in vitro anticancer drug release from mesoporous ...
2010-09-15 [J. Am. Chem. Soc. 132(36) , 12690-7, (2010)] |
|
Use of an internal reference for the quantitative HPLC-UV an...
2013-05-13 [ACS Comb. Sci. 15(5) , 229-234, (2013)] |
|
Toward quantifying the electrostatic transduction mechanism ...
2012-09-05 [J. Am. Chem. Soc. 134(35) , 14318-14321, (2012)] |
|
Effective purification of single-walled carbon nanotubes wit...
2010-05-18 [Langmuir 26(10) , 7561-7564, (2010)] |
|
Thioether Amide Based-Fluorescent Chemosensors for Pd2+...
[Bull. Korean Chem. Soc. 35(7) , 2189, (2014)] |