Metabolism and excretion of cysteinyl-leukotrienes.
S Hammarström, L Orning, K Bernström
文献索引:Adv. Prostaglandin. Thromboxane. Leukot. Res. 16 , 383-96, (1986)
全文:HTML全文
摘要
In vitro and in vivo experiments have demonstrated that a major pathway of metabolism of the glutathione containing leukotrienes involves modifications of the tripeptide substituent. The metabolic alterations are initiated by elimination of the N-terminal gamma-glutamyl residue, catalyzed by the enzyme gamma-glutamyl transferase. This reaction is followed by hydrolysis of the remaining peptide bond resulting in elimination of the C-terminal glycine residue. The enzyme catalyzing the latter reaction is a membrane bound dipeptidase which occurs in kidney and other tissues. The product formed by these reactions, leukotriene E4, has been tentatively identified as a urinary metabolite in man following intravenous administration of leukotriene C4. In rats, two major fecal metabolites of leukotriene C4 were characterized as having the structures N-acetyl leukotriene E4 and N-acetyl 11-trans leukotriene E4. These compounds are formed from leukotriene E4 and 11-trans leukotriene E4 in reactions with acetyl coenzyme A. A membrane bound enzyme, present in liver, kidney and other tissues, catalyzes these reactions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
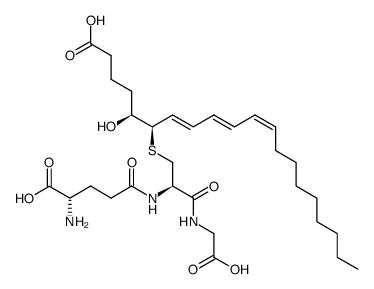 |
白三烯C3
CAS:77209-77-9 |
C30H49N3O9S |
|
Conversion of 5,8,11-eicosatrienoic acid to leukotrienes C3 ...
1981-03-10 [J. Biol. Chem. 256 , 2275, (1981)] |
|
Contractile activities of structural analogs of leukotrienes...
1981-05-01 [Proc. Natl. Acad. Sci. U. S. A. 78 , 3195, (1981)] |
|
Distribution and metabolism of 3H-labeled leukotriene C3 in ...
1982-01-10 [J. Biol. Chem. 257(1) , 531-5, (1982)] |
|
Metabolism of leukotriene C3 in the guinea pig. Identificati...
1981-09-25 [J. Biol. Chem. 256(18) , 9573-8, (1981)] |
|
Contractile activities of several cysteine-containing leukot...
2009-01-01 [Eur. J. Pharmacol. 86(2) , 207-15, (1982)] |