Journal of the American Chemical Society
2012-04-11
Site-selective bromination of vancomycin.
Tejas P Pathak, Scott J Miller
文献索引:J. Am. Chem. Soc. 134(14) , 6120-3, (2012)
全文:HTML全文
摘要
We report the site-selective bromination of vancomycin to produce, with substantial efficiency, previously unknown monobromovancomycins, a dibromovancomycin, and a tribromovancomycin. We document the inherent reactivity of native vancomycin toward N-bromophthalimide. We then demonstrate significant rate acceleration and perturbation of the inherent product distribution in the presence of a rationally designed peptide-based promoter. Alternative site selectivity is observed as a function of solvent and replacement of the peptide with guanidine.© 2012 American Chemical Society
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
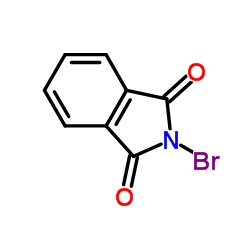 |
N-溴酞亚胺
CAS:2439-85-2 |
C8H4BrNO2 |
相关文献:
更多...
|
Enantioselective synthesis of multisubstituted biaryl skelet...
2013-03-13 [J. Am. Chem. Soc. 135(10) , 3964-70, (2013)] |
|
Titrimetric determination of acetylenic hyponotics using org...
1988-04-22 [Pharm. Weekbl. Sci. 10(2) , 90-2, (1988)] |
|
N-bromoimide/DBU combination as a new strategy for intermole...
2013-10-18 [Org. Lett. 15(20) , 5186-9, (2013)] |
|
Colorimetric and titrimetric assay of isoniazid.
1992-06-01 [J. Pharm. Biomed. Anal. 10(6) , 421-6, (1992)] |
|
C2-symmetric cyclic selenium-catalyzed enantioselective brom...
2013-01-30 [J. Am. Chem. Soc. 135(4) , 1232-5, (2013)] |